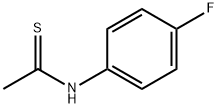
N-(4-FLUOROPHENYL)ETHANETHIOAMIDE synthesis
- Product Name:N-(4-FLUOROPHENYL)ETHANETHIOAMIDE
- CAS Number:351-84-8
- Molecular formula:C8H8FNS
- Molecular Weight:169.22
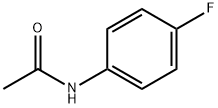
351-83-7
203 suppliers
$10.00/1g

351-84-8
6 suppliers
inquiry
Yield: 73%
Reaction Conditions:
with Lawessons reagent in tetrahydrofuran at 20; for 24 h;Inert atmosphere;
Steps:
1.1.2. General procedure B
General procedure: Compounds 2a-2e, 4a, and 4b were synthesized using a modified procedure.1 Compound 1a-1e, 3a, or 3b (1.0 equiv.) was added to a round-bottom flask. The flask was fitted with a rubber septum, purged with nitrogen gas, then dry THF was added. Lawesson’s reagent (0.7 equiv.) was added then the mixture was stirred at room temperature for 24 h and monitored by TLC. Upon completion the reaction mixture was quenched with H2O and the resulting mixture was extracted with EtOAc. The combined organic layers were washed with brine, dried over anh. Na2SO4, filtered then concentrated in vacuo. The crude mixture was purified by silica gel column chromatography to give the product.
References:
Lasing, Thitiya;Phumee, Atchara;Siriyasatien, Padet;Chitchak, Kantima;Vanalabhpatana, Parichatr;Mak, Kit-Kay;Hee Ng, Chew;Vilaivan, Tirayut;Khotavivattana, Tanatorn [Bioorganic and Medicinal Chemistry,2020,vol. 28,# 1,art. no. 115187] Location in patent:supporting information
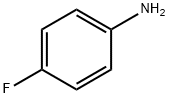
371-40-4
403 suppliers
$5.00/5 g

351-84-8
6 suppliers
inquiry