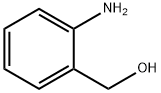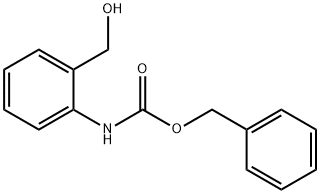
BENZYL 2-(HYDROXYMETHYL)PHENYLCARBAMATE synthesis
- Product Name:BENZYL 2-(HYDROXYMETHYL)PHENYLCARBAMATE
- CAS Number:111881-64-2
- Molecular formula:C15H15NO3
- Molecular Weight:257.28
Yield:111881-64-2 100%
Reaction Conditions:
with sodium carbonate in tetrahydrofuran;water at 0; for 0.5 h;
Steps:
N-benzyloxycarbonyl-2-aminobenzyl alcohol.[3]
A 250-mL 3-necked round-bottom flask was charged with 2-aminobenzyl alcohol (2.5 g, 20 mmol, 1.0 equiv), anhydrous tetrahydrofuran (THF; 10 mL), and aqueous saturated sodium carbonate (65 mL). The reaction was cooled to 0 °C and benzyl chloroformate (3.30 mL, 22 mmol, 1.1 equiv) and additional aqueous saturated sodium carbonate (35 mL) were added simultaneously. The reaction was stirred at 0 °C for 30 min., after which the solution was a cloudy white with some brown solid settling to the bottom. Ethyl acetate (ca. 60 mL) was added, and the layers were partitioned. The clear brown organic layer was washed with brine (20 mL) and dried over anhydrous sodium sulfate. Solvent was removed in vacuo, and the resulting solid was dried under vacuum overnight. The resulting white solid (5.25 g, quantitative yield) was used without further purification. TLC conditions: 15% ethyl acetate in hexanes; Rf = 0.38; UV visualization. Analytical information was found to correspond to literature values.3 1H-NMR (300 MHz, CDCl3, 20 °C) δ 7.98 (2H, br s, NH), 7.44-7.31 (5H, m, ArH), 7.17 (2H, d, J = 7.5 Hz, ArH), 7.04 (2H, t, J = 7.5 Hz, ArH), 5.21 (2H, s, CH2), 4.70 (2H, d, J = 5.7 Hz, CH2O).
References:
Wagner, Anna M.;Knezevic, Claire E.;Wall, Jessica L.;Sun, Victoria L.;Buss, Joshua A.;Allen, Leeann T.;Wenzel, Anna G. [Tetrahedron Letters,2012,vol. 53,# 7,p. 833 - 836] Location in patent:supporting information; experimental part
552-89-6
612 suppliers
$5.00/1g

111881-64-2
13 suppliers
inquiry
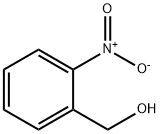
612-25-9
314 suppliers
$6.00/5g

111881-64-2
13 suppliers
inquiry
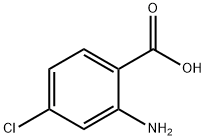
89-77-0
418 suppliers
$6.00/10g

111881-64-2
13 suppliers
inquiry