
Benzyl (4R)-1-Boc-4-hydroxy-D-prolinate synthesis
- Product Name:Benzyl (4R)-1-Boc-4-hydroxy-D-prolinate
- CAS Number:132622-92-5
- Molecular formula:C17H23NO5
- Molecular Weight:321.37

100-39-0
423 suppliers
$10.00/10g
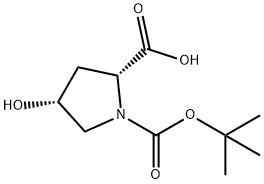
135042-12-5
186 suppliers
$6.00/1g

132622-92-5
10 suppliers
inquiry
Yield:132622-92-5 100%
Reaction Conditions:
Stage #1: (4R)-1-(tert-butoxycarbonyl)-4-hydroxy-D-prolinewith caesium carbonate in methanol;water at 0; for 0.5 h;
Stage #2: benzyl bromide in N,N-dimethyl acetamide at 20;
Steps:
14.2 2-benzyl 1-tert-butyl (2R,4R)-4-hydroxypyrrolidine-1,2-dicarboxylate
At room temperature,15a (8.6 g, 37.21 mmol) was dissolved in methanol (80 mL), cooled to 0 ° C,A solution of cesium carbonate (6.06 g, 18.61 mmol) in water (42 mL) was added dropwise to the reaction solution,Stirred for 30 minutes and concentrated under reduced pressure to remove the solvent to give a white solid.The residue was dissolved in N, N-dimethylformamide (68 mL), benzyl bromide (6.36 g, 37.21 mmol) was added and the reaction was stirred at room temperature overnight.Most of the solvent was removed by distillation under reduced pressure, water (100 mL) was added, and extracted with ethyl acetate (100 mL x 3).The organic phases were combined and washed successively with water (100 mL x 1), saturated brine solution (100 mL x 2).Dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure to give a pale yellow liquid 15b (11.95 g, yield 100%).
References:
TW2017/8221,2017,A Location in patent:Page/Page column 92; 93; 94

24424-99-5
826 suppliers
$13.50/25G

132622-89-0
0 suppliers
inquiry

132622-92-5
10 suppliers
inquiry

2584-71-6
325 suppliers
$6.00/1g

132622-92-5
10 suppliers
inquiry

24424-99-5
826 suppliers
$13.50/25G

132622-92-5
10 suppliers
inquiry
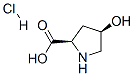
77449-94-6
163 suppliers
$10.00/1g

132622-92-5
10 suppliers
inquiry