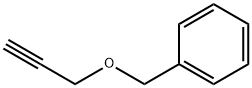
BENZYL PROPARGYL ETHER synthesis
- Product Name:BENZYL PROPARGYL ETHER
- CAS Number:4039-82-1
- Molecular formula:C10H10O
- Molecular Weight:146.19
14593-43-2
123 suppliers
$18.00/1g

4039-82-1
60 suppliers
$30.50/5g
Yield: 96%
Reaction Conditions:
Stage #1:benzyl allyl ether with pyridinium perbromide hydrobromide in acetonitrile at 20; for 12 h;Molecular sieve;Inert atmosphere;
Stage #2: with tetra(n-butyl)ammonium hydroxide;dimethyl sulfoxide;triethylamine in water;acetonitrile at 0 - 60; for 2 h;Molecular sieve;Inert atmosphere;Reagent/catalyst;
Steps:
One-Pot Synthesis of 4 by Method B; General Procedure
General procedure: A mixture of allyl alcohol derivative 1 (x g, 1.0 equiv), py·HBr3 (1.1equiv), and MS 13X (ca. 10x g) in MeCN (y mL, 0.1 M) was stirred at r.t. for 12-14 h. Then, DMSO (7y mL), TBAOH (3.1 equiv, 40% in H2O),and Et3N (1.1 equiv) were added to the reaction mixture at 0 °C and the system was heated to 60 °C. The reaction was quenched with sat.aq NH4Cl at 0 °C. After the removal of MS 13X through a cotton filter, the resulting filtrate was extracted with hexane-EtOAc (2:1, 3 × 30mL) and dried (MgSO4). The combined extracts were concentrated under reduced pressure, and the residue was purified by column chromatography (silica gel) to afford the propargyl alcohol derivative 4.
References:
Kutsumura, Noriki;Inagaki, Mai;Kiriseko, Akito;Saito, Takao [Synthesis,2015,vol. 47,# 13,art. no. SS-2015-F0081-OP,p. 1844 - 1850]

106-96-7
339 suppliers
$30.00/25g

100-51-6
1415 suppliers
$5.00/100g

4039-82-1
60 suppliers
$30.50/5g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

4039-82-1
60 suppliers
$30.50/5g


