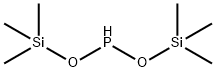
BIS(TRIMETHYLSILYLOXY)-PHOSPHINE synthesis
- Product Name:BIS(TRIMETHYLSILYLOXY)-PHOSPHINE
- CAS Number:30148-50-6
- Molecular formula:C6H19O2PSi2
- Molecular Weight:210.36
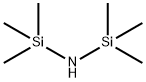
999-97-3
654 suppliers
$9.00/5G

30148-50-6
1 suppliers
inquiry
Yield:30148-50-6 85%
Reaction Conditions:
with ammonium hypophosphorous acid at 120 - 130; for 2 h;Inert atmosphere;
Steps:
Bis(trimethylsilyl) phosphonite (11)
Ammonium hypophosphite 10 (1.05 g, 12.65 mmol) was placed in a three-neck round-bottomed flask fitted with a septum, thermometer, reflux condenser, and a bubbler, and was purged on the nitrogen line. Hexamethyldisilazane (HMDS) (2.77 cm3, 2.14 g, 13.28 mmol) was injected, and the temperature was raised to 120-130 °C with stirring for two hours until the evolution of ammonia (NH3) had ceased. Cessation of ammonia evolution was monitored by moist pH paper at a bubbler on the nitrogen line. The reaction was then fractionally distilled under reduced pressure. The product 11 was collected at 25-30 °C/2.5 mm Hg as a colourless oil (2.26 g, 85%). The product was not stored, owing to its pyrophoric nature, but was used straight away in subsequent reactions. 1H NMR (400 MHz, C6D6): δH 7.55 (1H, d, 1JP-H = 176.1 Hz, PH), 0.04 (18H, s, 2 x Si(CH3)3).13C NMR (100 MHz, C6D6): δC 0.02 (d, 3JC-P = 2.9 Hz, POSiCH3).31P NMR (162 MHz, C6D6, proton-coupled): δP 157.06 (d, 1JP-H = 176.1 Hz, PH).
References:
Markoulides, Marios S.;Regan, Andrew C. [Tetrahedron Letters,2011,vol. 52,# 23,p. 2954 - 2956] Location in patent:supporting information; experimental part
10416-59-8
580 suppliers
$18.00/5g

30148-50-6
1 suppliers
inquiry
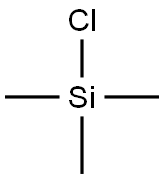
75-77-4
593 suppliers
$5.04/10

30148-50-6
1 suppliers
inquiry
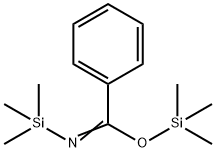
18412-35-6
0 suppliers
inquiry

30148-50-6
1 suppliers
inquiry
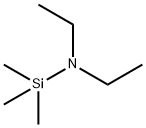
996-50-9
224 suppliers
$15.00/25g

30148-50-6
1 suppliers
inquiry