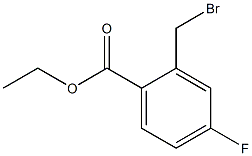
BIWXELAKQASQHM-UHFFFAOYSA-N synthesis
- Product Name:BIWXELAKQASQHM-UHFFFAOYSA-N
- CAS Number:343942-94-9
- Molecular formula:C10H10BrFO2
- Molecular Weight:261.0876
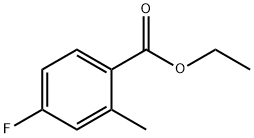
167758-88-5
38 suppliers
$18.00/5g

343942-94-9
0 suppliers
inquiry
Yield:343942-94-9 90%
Reaction Conditions:
with dihydrogen peroxide;bromine in dichloromethane;water at 20; for 24 h;
Steps:
2
Compound 2b (1.82 g, 10 mmol)Dissolve in dichloromethane (15 mL), add water (15 mL), and stir at room temperature.Bromine (513 μL, 10 mmol) and 30% hydrogen peroxide (1 mL) were added dropwise in sequence,Continue stirring and react for 24 hours.15 mL of dichloromethane was added, shaken evenly, and the organic layer was separated; the aqueous layer was extracted with dichloromethane (15 mL×2). Combine the organic phases,Washed with saturated saline (30 mL×2),dried over anhydrous sodium sulfate, filtered,The filtrate was concentrated under reduced pressure to obtain a yellow liquid.Purified by silica gel column chromatography (petroleum ether/ethyl acetate (V/V)=40/1) to obtain 2.32 g of colorless liquid compound 2c, yield: 90.0%.
References:
CN113880832,2022,A Location in patent:Paragraph 0109-0110

321-21-1
234 suppliers
$6.00/1g

343942-94-9
0 suppliers
inquiry