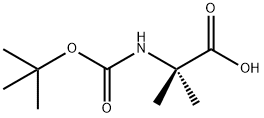
Boc-Aib-OH synthesis
- Product Name:Boc-Aib-OH
- CAS Number:30992-29-1
- Molecular formula:C9H17NO4
- Molecular Weight:203.24

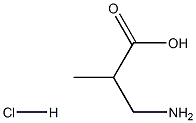
24424-99-5
834 suppliers
$13.50/25G
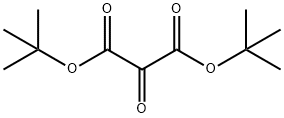
62-57-7
420 suppliers
$10.64/25G

30992-29-1
268 suppliers
$6.00/5g
Yield:30992-29-1 90%
Reaction Conditions:
with sodium hydroxide in 1,4-dioxane;water at 20; for 16 h;Cooling with ice;
Steps:
2-(1-(((tert-butoxycarbonyl) amino) methyl) cyclohexyl) aceticacid (3a)
General procedure: To a stirred solution of 2-(1-(aminomethyl) cyclohexyl)acetic acid 2a (2g, 11.68 mmol, 1equiv.) in 1, 4-dioxane (20 mL) atice temperature, aq. NaOH (560.57mg, 14.02 mmol, 1.2 equiv,dissolved in 10 mL water) was added followed by addition of ditert-butyl dicarbonate (5.10 gm, 23.36mmol, 2 equiv.) in 1,4-dioxane (5mL). The reaction mixture was stirred at room temperature for 16 h. The resulting suspension was concentrated in vacuo and the residuewas dissolved in water (10-15mL) and washed by ethyl acetate (15mL x 2). The resultingaqueous layer was acidified using dilute KHSO4 solution, followed by extraction withethyl acetate (30 mL x 3), dried (Na2SO4) and concentrated in vacuo to afford 3a as awhite solid, which was carried forward for the next reaction. Yield: 3.04g, 95%.
References:
Dangi, Abha;Marelli, Udaya Kiran;Meena, Chhuttan L.;Reichart, Florian;Sanjayan, Gangadhar J.;Singh, Dharmendra;Zahler, Stefan;Weinmüller, Michael [Bioorganic and medicinal chemistry letters,2020] Location in patent:supporting information
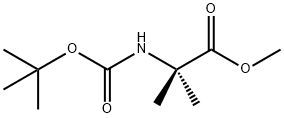
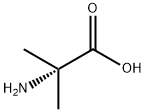
84758-55-4
39 suppliers
$39.00/100mg

30992-29-1
268 suppliers
$6.00/5g
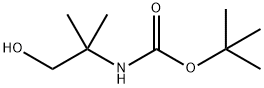
102520-97-8
116 suppliers
$7.00/1g

30992-29-1
268 suppliers
$6.00/5g