
BROMODIFLUOROACETIC ACID synthesis
- Product Name:BROMODIFLUOROACETIC ACID
- CAS Number:354-08-5
- Molecular formula:C2HBrF2O2
- Molecular Weight:174.93
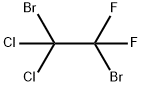
558-57-6
91 suppliers
$20.00/1 g
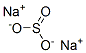
7757-83-7
692 suppliers
$13.50/250G

354-08-5
139 suppliers
$12.00/1 g
Yield:-
Reaction Conditions:
with sulfur trioxide in water
Steps:
2 Production of Bromodifluoroacetic Acid from CF2BrCCl2Br:
Example 2 Production of Bromodifluoroacetic Acid from CF2BrCCl2Br: 153 g (0.5 mol) of 1,2-dibromo-1,1-difluoro-2,2-dichloroethane obtained above are loaded into a first glass reactor of 500 cm3, provided with mechanical stirring, an isobaric dropping funnel and a distillation column surmounted with a timer for adjusting the refluxing rate. The draw-off of the column is connected to a second reactor containing 150 g of water. This reactor is cooled by means of a jacket (5° C.) and is provided with mechanical stirring and a condenser connected to a water absorber. The reagent in the first reactor is heated to 50° C., and then 123 g of oleum 65% are poured in over 2 h 30 min, which corresponds to 1 mol of SO3. The distilling fraction drawn off continuously, which contains bromodifluoroacetyl halide, is hydrolyzed in the second reactor whose temperature is maintained at around 25° C. At the end of the reaction, the apparatus is flushed with nitrogen and cooled to room temperature. A 7% sodium sulphite solution is added to the content of the second reactor so as to destroy the traces of bromine. The bromodifluoroacetic acid is then extracted with isopropyl ether and then distilled under reduced pressure. The yield of bromodifluoroacetic acid relative to the perhalogenated derivative is 80%.
References:
ATOFINA US2003/32836, 2003, A1
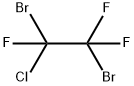
354-51-8
102 suppliers
$15.00/5 g

354-08-5
139 suppliers
$12.00/1 g

667-27-6
410 suppliers
$10.00/10 g

354-08-5
139 suppliers
$12.00/1 g
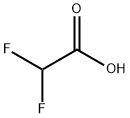
381-73-7
312 suppliers
$10.00/1g

354-08-5
139 suppliers
$12.00/1 g