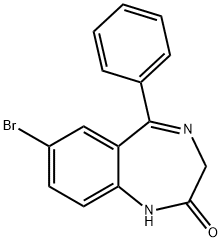
Bromonordiazepam synthesis
- Product Name:Bromonordiazepam
- CAS Number:2894-61-3
- Molecular formula:C15H11BrN2O
- Molecular Weight:315.16
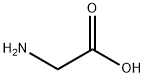
56-40-6
1338 suppliers
$5.00/25g

34099-70-2
2 suppliers
inquiry

612527-22-7
0 suppliers
inquiry

2894-61-3
0 suppliers
inquiry
Yield:612527-22-7 67%
Reaction Conditions:
with water;sodium carbonate in ethanol; for 5 h;Heating / reflux;
Steps:
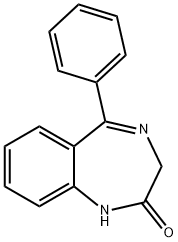
suspension of 7-bromo-1,3-dihydro-5-phenyl-2H-1,4-benzodiazepin-2-thione 84 (1.6 g, 4.83 mmol), glycine (1.81 g, 24.2 mmol) and Na2CO3 (1.84 g, 17.4 mmol) in EtOH (38 mL)-H2O (16 mL) was stirred at reflux for 5 h, poured into water (100 mL), and then filtered to remove a small amount of 7-bromo-1,3-dihydro-5-phenyl-2H-1,4-benzodiazepin-2-one which remained. See Archer G A, Sternbach L H (1964) J Org Chem 29:231. The filtrate was extracted with CHCl3. The CHCl3 extract was discarded; the aqueous layer was adjusted to pH 4 with 2N HCl and then extracted with CHCl3 (3×25 mL). Evaporation of the CHCl3 solution gave pure acid 85 (1.2 g, 67%) as a yellow solid. Acid 85 (350 mg, 0.941 mmol) was suspended in dry CH2Cl2 (10 mL) and DCC (223 mg, 1.08 mmol) was added. The suspension which resulted was stirred at 40° C. for 2 h and then cooled to 0° C. It was filtered, and the solvent was removed under vacuum to give 8-bromo-2,4-dihydro-6-phenyl-1H-imidazo[1,2-a][1,4]benzodiazepin-1-one 86 as a brown oil. The cyclized product 86 (ca. 250 mg) was dissolved in dry benzene (6 mL), dimethylformamide diethylacetal (130 mg, 0.883 mmol) and triethylamine (89 mg, 0.883 mmol) were added. The solution which resulted was stirred at room temperature for 1 h and the solvent was removed under vacuum, The residue was then crystallized from EtOAc-MeOH to give 87 (200 mg, 70%). A solution of 87 (180 mg, 0.440 mmol) in dry toluene (5 mL) was treated with 1-methyl piperazine (1 mL) and heated to reflux for 5 h. The solvent was removed in vacuum to give a gum which crystallized from CH2Cl2-Et2O to furnish 88 (PS-I-35, 146 mg, 72%). mp>250° C.; IR (KBr) 3324, 2932, 2787, 1692, 1624, 1475, 1402, 1297, 1137, 933 cm-1; 1H NMR (CDCl3) δ 7.95 (d, 1H, J=8.8 Hz), 7.72 (dd, 1H, J=2.3 Hz, J=8.8 Hz), 7.58-7.55 (m, 2H), 7.49-7.37 (m, 4H), 7.17 (s, 1H), 5.01 (d, 1H, J=12 Hz), 4.50-4.60 (m, 1H), 4.20-4.30 (m, 1H), 4.16 (d, 1H, J=12 Hz), 3.50-3.58 (m, 2H), 2.40-2.60 (m, 4H), 2.34 (s, 3H); MS (m/z) 465 (100). Anal. Calcd. for C23H22N5OBr.H2O: C, 58.79; H, 4.95; N, 14.89. Found: C, 58.73; H, 4.86; N,
References:
US2006/3995,2006,A1 Location in patent:Page/Page column 48-49
2898-08-0
89 suppliers
$80.00/1g

2894-61-3
0 suppliers
inquiry
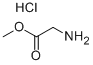
5680-79-5
546 suppliers
$3.66/25g
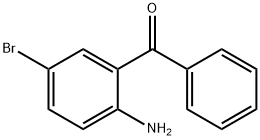
39859-36-4
152 suppliers
$9.00/250mg

2894-61-3
0 suppliers
inquiry

39859-36-4
152 suppliers
$9.00/250mg

2894-61-3
0 suppliers
inquiry
