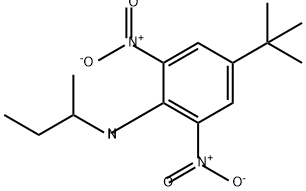
BUTRALIN synthesis
- Product Name:BUTRALIN
- CAS Number:33629-47-9
- Molecular formula:C14H21N3O4
- Molecular Weight:295.33
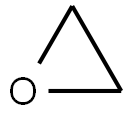
75-21-8
218 suppliers
$33.29/crm44609
4097-49-8
85 suppliers
$34.00/1g

33629-47-9
83 suppliers
$50.00/250mg
Yield:33629-47-9 94%
Reaction Conditions:
with 1-methyl-1H-imidazole in toluene
Steps:
II EXAMPLE II
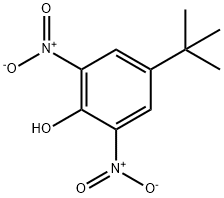
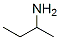
EXAMPLE II To a container was added 20 grams (0.13 mole) of 2,6-dinitro-4-tert-butylphenol, 10 drops of N-methylimidazole, 30 grams of ethylene oxide and 200 ml. of anhydrous toluene. The resulting mixture was transferred to a 600 ml. Parr reactor. The mixture was heated and stirred under pressure to 150° C. and maintained at that temperature for a three to four hour period. After this reaction period, 97 percent of the starting 2,6-dinitro-4-tert-butylphenol was found to be converted into 2-(2,6-dinitro-4-butylphenoxy) ethanol according to liquid-liquid chromatographic analysis. The reaction mixture was then evaporated under reduced pressure to give 23 grams of a dark brown ethoxylated material. The yield of ethoxylated material was almost quantitative based upon 2,6-dinitro-4-tert-butylphenol reactant. To a reactor was added 18.5 grams (0.065 mole) of the above ethoxylated material and 12 grams of secondary butylamine. The resulting mixture was stirred and heated to between 50° C. and 60° C. for 30 minutes. After cooling, the reaction mixture was poured into ice-water to crystallize the product. The product was recovered by filtration and vacuum-dried to give 18 grams of N-sec-butyl-2,6-dinitro-4 -tert-butylaniline having a melting point of 54° C. to 57° C. The yield was 94 percent based on the ethoxylated derivative reactant.
References:
Union Carbide Corporation US4289907, 1981, A
33966-50-6
63 suppliers
inquiry

4097-49-8
85 suppliers
$34.00/1g

98-59-9
584 suppliers
$9.00/5g

33629-47-9
83 suppliers
$50.00/250mg

33966-50-6
63 suppliers
inquiry

2213-81-2
44 suppliers
$56.00/50 mg

33629-47-9
83 suppliers
$50.00/250mg

33966-50-6
63 suppliers
inquiry
77055-30-2
52 suppliers
$45.00/250mg

33629-47-9
83 suppliers
$50.00/250mg

98-54-4
405 suppliers
$15.00/25g

33629-47-9
83 suppliers
$50.00/250mg