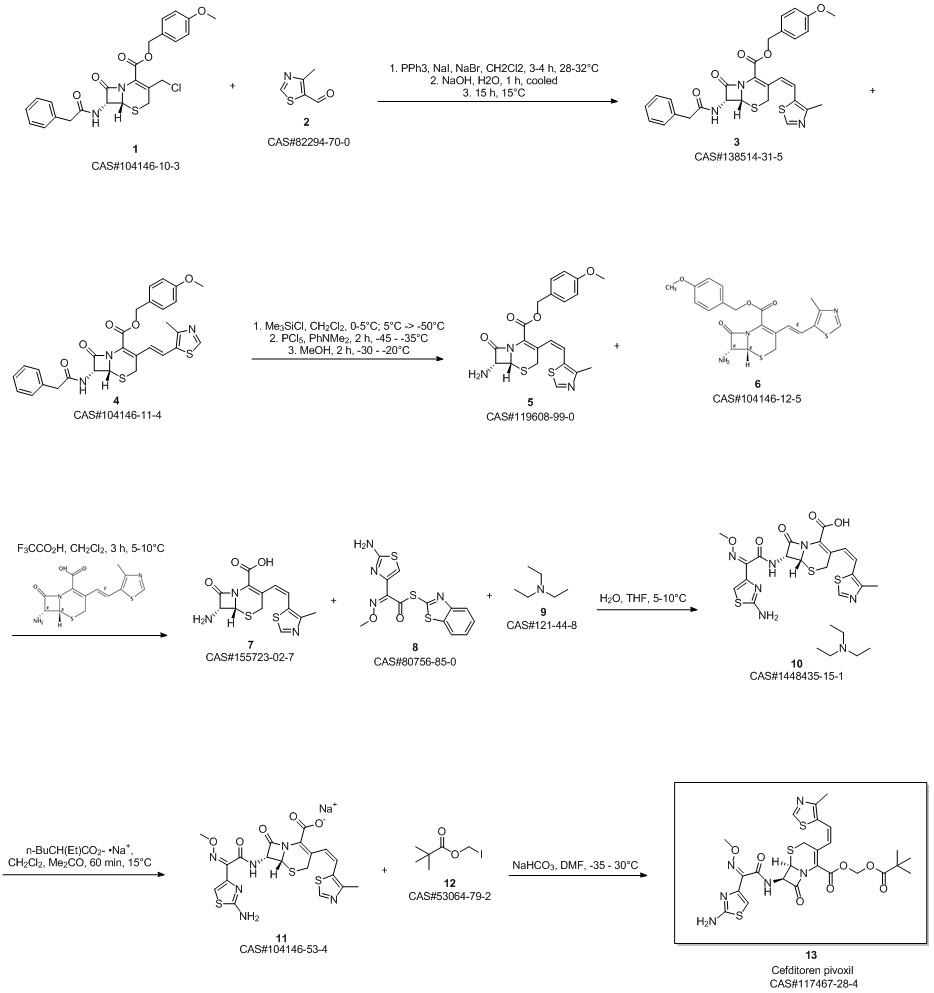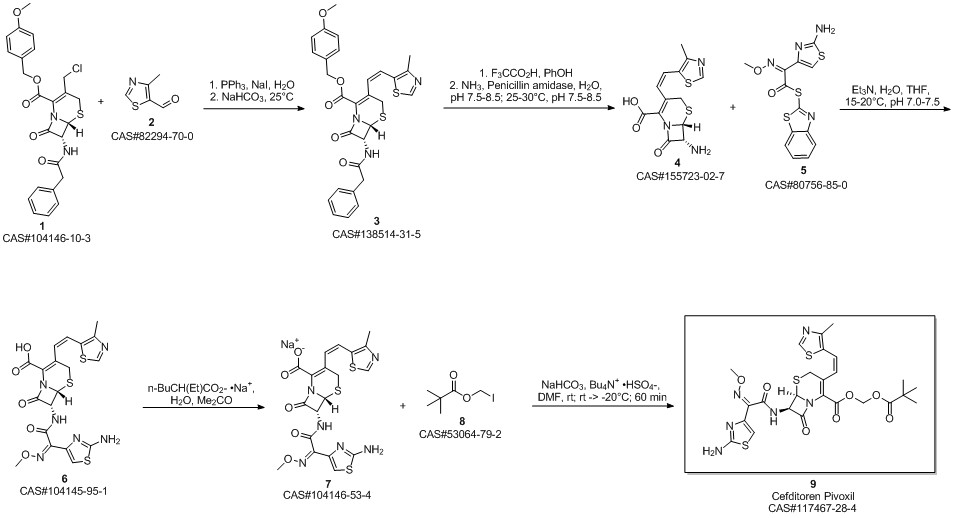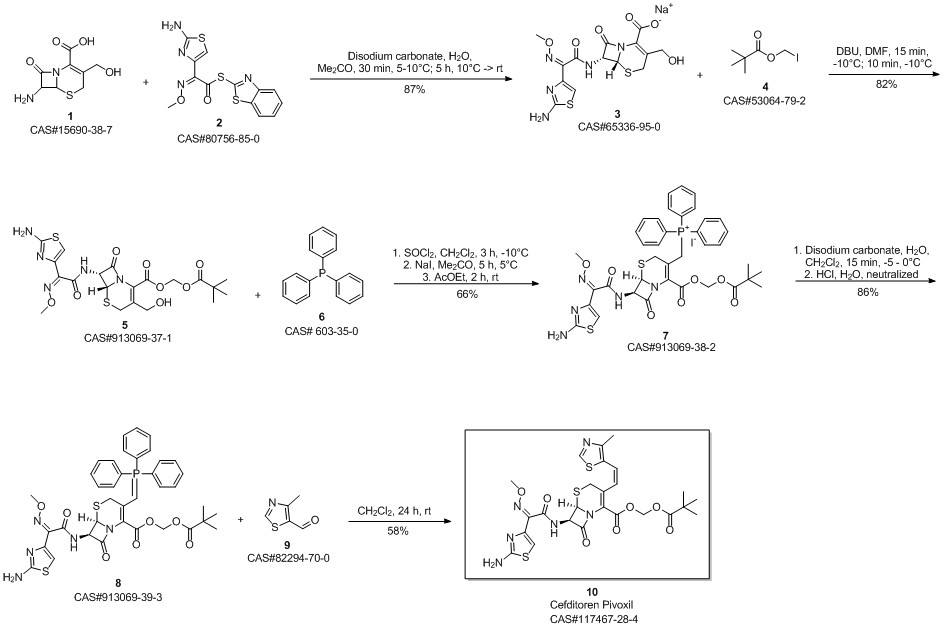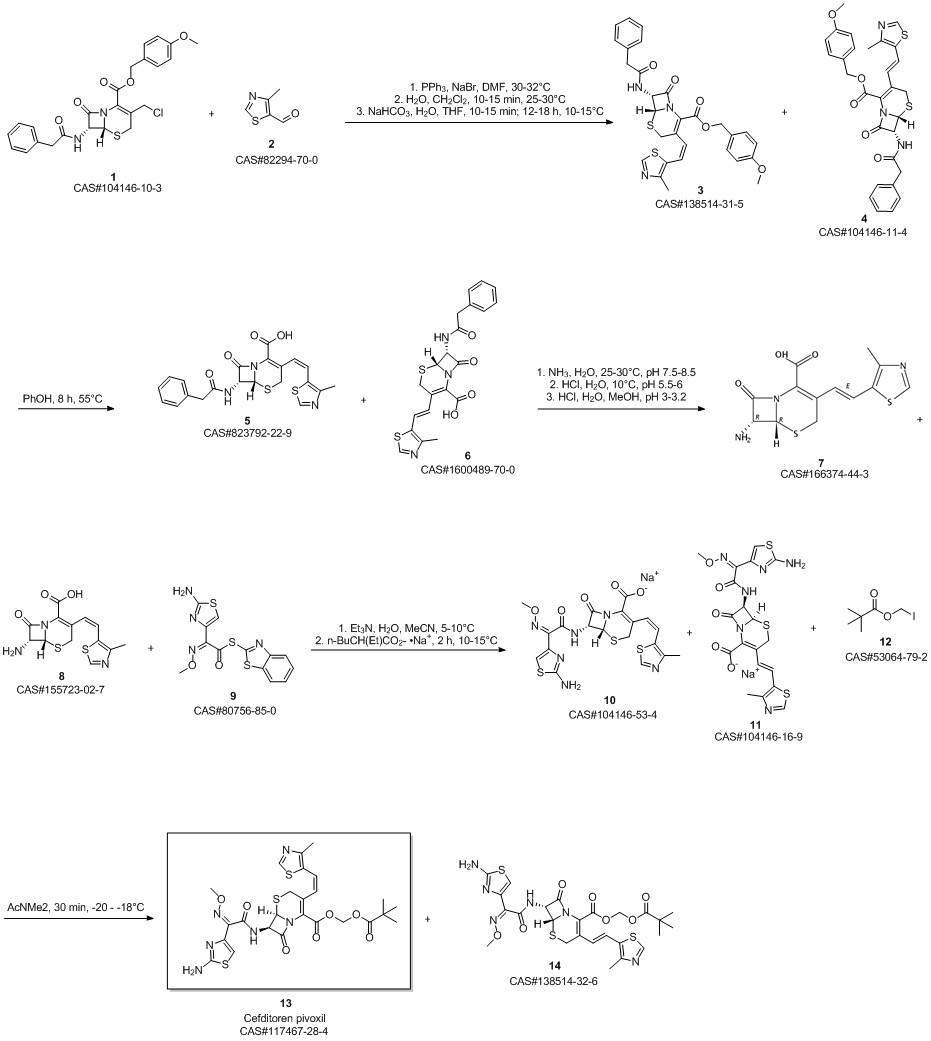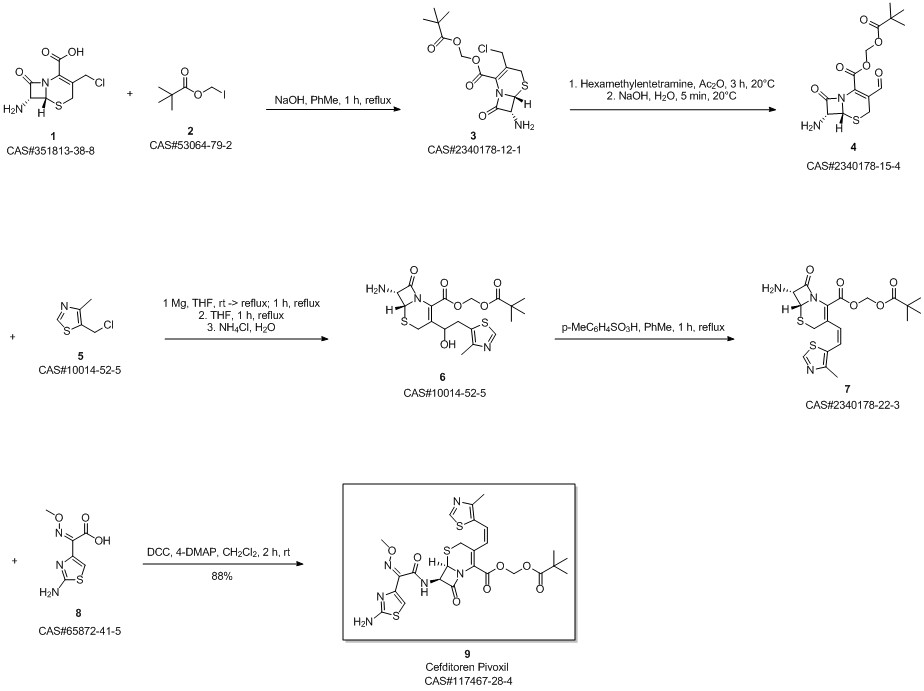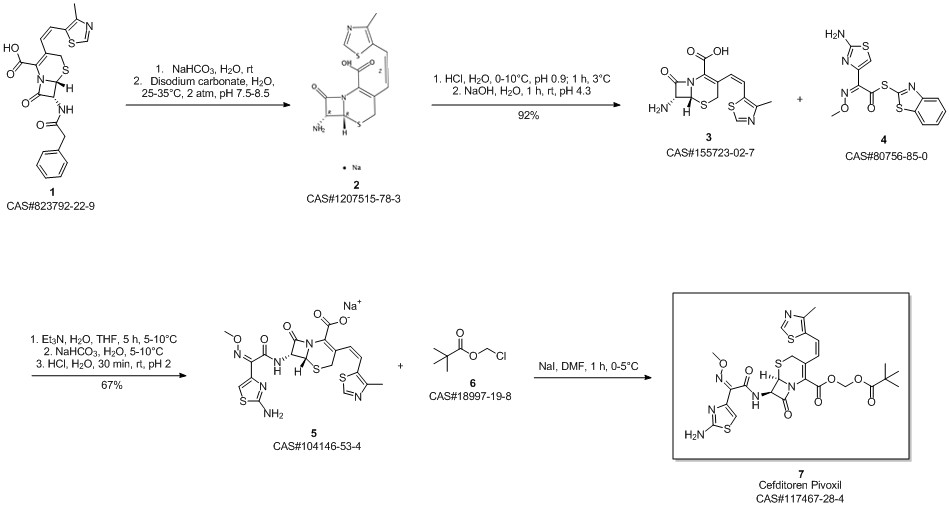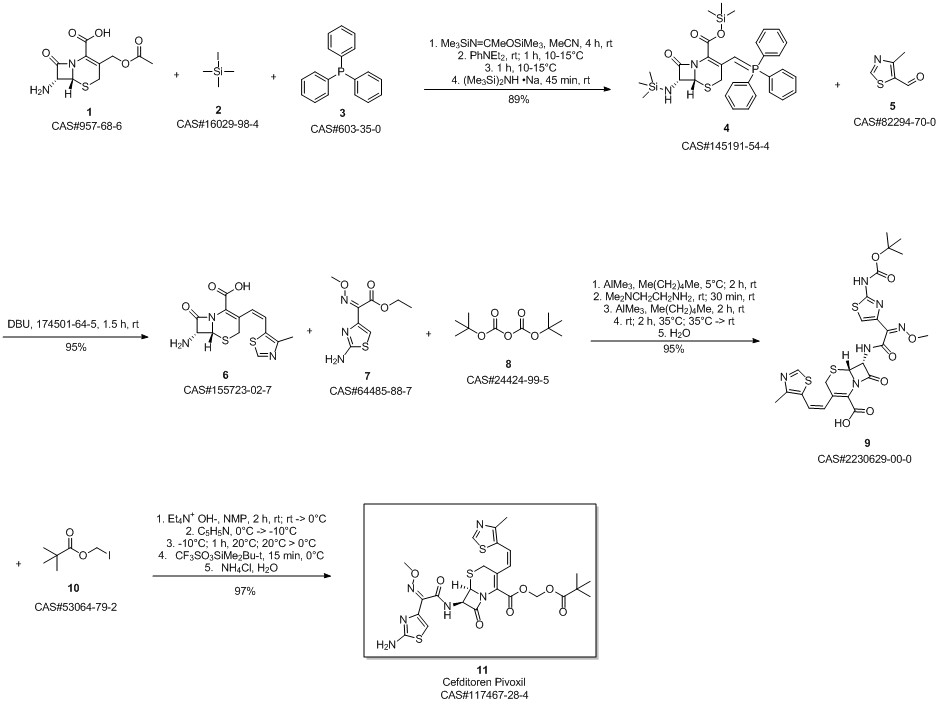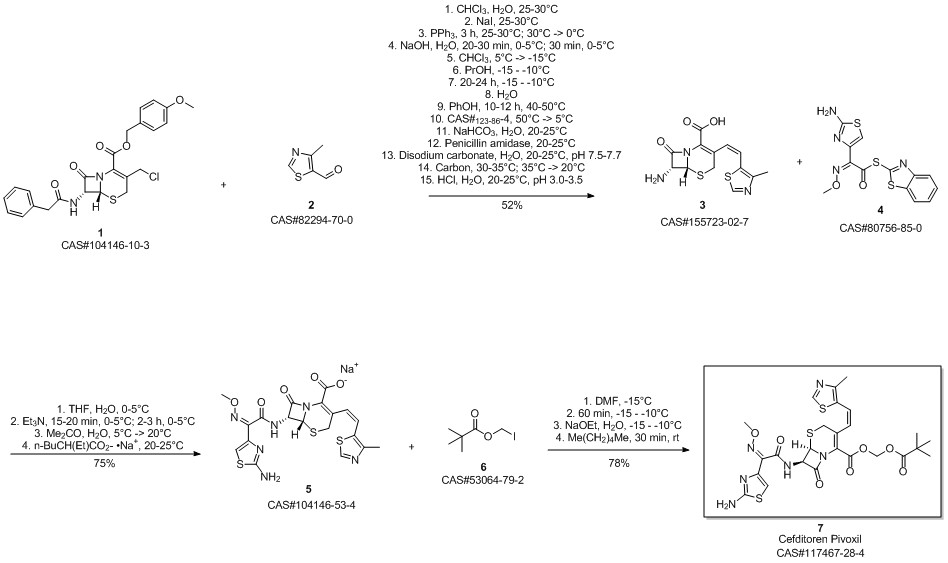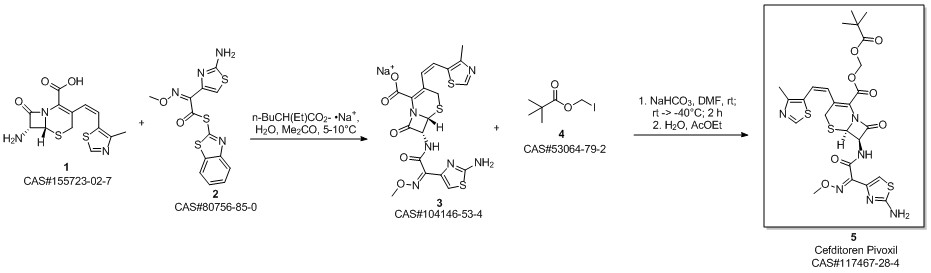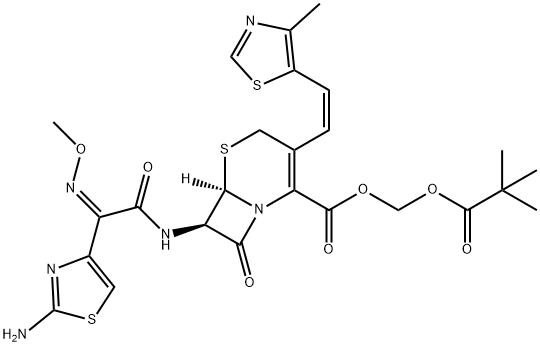
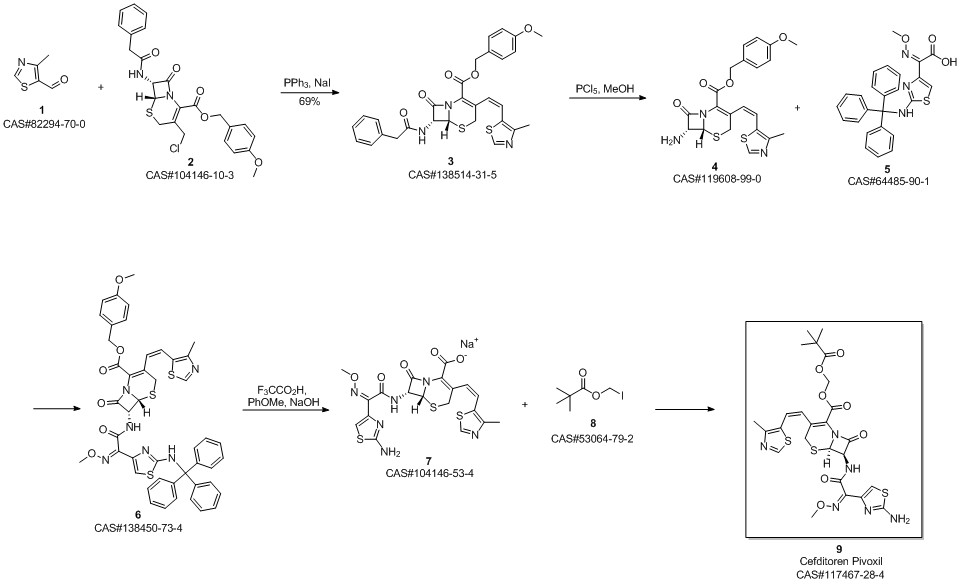
Cefditoren pivoxil synthesis
- Product Name:Cefditoren pivoxil
- CAS Number:117467-28-4
- Molecular formula:C25H28N6O7S3
- Molecular Weight:620.72
Sakagami, Kenji; Atsumi, Kunio; Yamamoto, Yuichi; Tamura, Atsuhi; Yoshida, Takashi; Nishihata, Ken; Fukatsu, Shunzo. Synthesis and oral activity of pivaloyloxymethyl 7-[(Z)-2-(2-aminothiazol-4-yl)-2-methoxyiminoacetamido]-3(Z)-(4-methylthiazol-5-yl)vinyl-3-cephem-4-carboxylate (ME1207) and its related compound. Chemical & Pharmaceutical Bulletin. Pharm. Res. Cent. Meiji Seika Kaisha, Ltd. Yokohama, Japan 222. Volume 39. Issue 9. Pages 2433-6. 1991
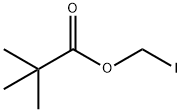
53064-79-2
192 suppliers
$19.00/1g

104145-95-1
47 suppliers
inquiry

117467-28-4
310 suppliers
$5.00/25mg
Yield:117467-28-4 98.75%
Reaction Conditions:
Stage #1: cefditorenwith pyridine in N,N-dimethyl-formamide at -10;
Stage #2: iodomethyl pivaloate at -10; for 1 h;
Steps:
9 Example 9: Preparation of Compound 1
Compound 2 (5.29 g, 10 mmol) and 60 mL of N,N-dimethylformamide were added to the reaction flask, and the mixture was stirred for 30 min.Cool down to 0 ° C or below 0 ° C,Add 8.5mmol of pyridine,Cool down to -10 ° C, join quickly20mmol iodomethyl pivalate,Insulation reaction for 1 h,Add activated carbon and stir for 30min, decolorization filtration,The filtrate was added with 5 g of anhydrous sodium sulfate.Add 30 mL of water and 60 mL of ethyl acetate.Stir, let stand,The aqueous phase was extracted once with ethyl acetate.Combine ethyl acetate,Evaporate the solvent under reduced pressure.Get oil,Add 10mL of acetone,40mL of isopropyl ether, crystallization, filtration,Drying cefoperapyl pure product 6.14g,The yield is 98.75%.The purity is 99.84%.
References:
CN109336904,2019,A Location in patent:Paragraph 0042; 0043

53064-79-2
192 suppliers
$19.00/1g
![sodium (6R,7R)-7-[[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-methoxyimino-acetyl]amino]-3-[(E)-2-(4-methyl-1,3-thiazol-5-yl)ethenyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid](/CAS/GIF/104146-53-4.gif)
104146-53-4
65 suppliers
$387.00/5mg

117467-28-4
310 suppliers
$5.00/25mg
82294-70-0
281 suppliers
$6.00/1g

117467-28-4
310 suppliers
$5.00/25mg

957-68-6
437 suppliers
$14.00/5g

117467-28-4
310 suppliers
$5.00/25mg

104146-10-3
258 suppliers
$5.00/5g

117467-28-4
310 suppliers
$5.00/25mg