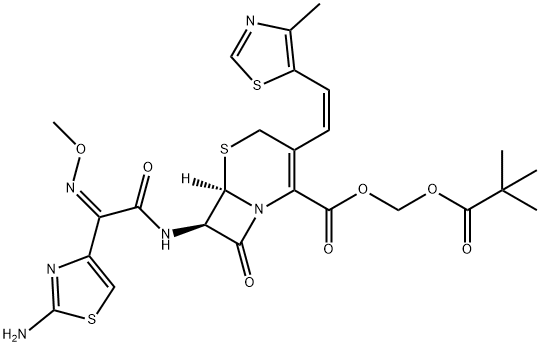
Cefditoren pivoxil
- CAS No.
- 117467-28-4
- Chemical Name:
- Cefditoren pivoxil
- Synonyms
- CEFDITOREN;Spectracef;CEFDITORIN PIVOXIL;Meiac;MEIACT;ME-1207;toubaotuolunzhi;toubaotuolkunzhi;ceditoren pivoxil;Cefditoren Pivoxi
- CBNumber:
- CB7377697
- Molecular Formula:
- C25H28N6O7S3
- Molecular Weight:
- 620.72
- MDL Number:
- MFCD00933166
- MOL File:
- 117467-28-4.mol
| Melting point | 207-209°C |
|---|---|
| alpha | D20 -48.5° (c = 0.5 in methanol) |
| Density | 1.55±0.1 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C |
| solubility | DMSO (Slightly), Ethanol (Slightly), Methanol (Slightly, Heated) |
| form | Solid |
| pka | 8.08±0.60(Predicted) |
| color | Off-White to Pale Yellow |
| Merck | 14,1921 |
| CAS DataBase Reference | 117467-28-4(CAS DataBase Reference) |
| FDA UNII | 78THA212DH |
| ATC code | J01DD16 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H317-H334 |
| Precautionary statements | P261-P285-P304+P341-P342+P311-P501-P261-P272-P280-P302+P352-P333+P313-P321-P363-P501 |
| RTECS | XI0367800 |
| HS Code | 2941.90.3000 |
Cefditoren pivoxil price More Price(39)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TCI Chemical | C2856 | Cefditoren Pivoxil >98.0%(HPLC)(T) | 117467-28-4 | 200mg | $248 | 2024-03-01 | Buy |
| Cayman Chemical | 30315 | Cefditoren Pivoxil | 117467-28-4 | 5mg | $68 | 2024-03-01 | Buy |
| Cayman Chemical | 30315 | Cefditoren Pivoxil | 117467-28-4 | 10mg | $121 | 2024-03-01 | Buy |
| Cayman Chemical | 30315 | Cefditoren Pivoxil | 117467-28-4 | 50mg | $201 | 2024-03-01 | Buy |
| Cayman Chemical | 30315 | Cefditoren Pivoxil | 117467-28-4 | 100mg | $268 | 2024-03-01 | Buy |
Cefditoren pivoxil Chemical Properties,Uses,Production
Description
Cefditoren pivoxil is an orally bioavailable prodrug form of the broad-spectrum cephalosporin antibiotic cefditoren. Cefditoren pivoxil is hydrolyzed by intestinal wall esterases to form cefditoren. Cefditoren pivoxil is effective against systemic S. aureus, E. coli, K. pneumoniae, P. mirabilis, or S. marcescens infections in mice with ED50 values of 10, 2.5, 11, 4.4, and 11 mg/kg, respectively. Formulations containing cefditoren pivoxil were previously used in the treatment of acute bacterial exacerbations of chronic bronchitis and community-acquired pneumonia.
Description
Cefditoren pivoxil is an orally active third generation cephalosporin introduced in Japan as a treatment for a broad range of bacterial infections including dermatological and other community acquired infections. Cefditoren pivoxil is reported to have a broad spectrum of activity against both Gram-positive and Gramnegative bacteria, more potent than many other existing agents of its class. In particular, it shows the highest therapeutic activity against S. pneumoniae and S. marcescens infections. It exhibits resistance to β-lactamase hydrolysis typical of third generation cephalosporins. As a prodrug of cefditoren, it is readily absorbed through GI tract and has low toxicity and side effects.
Chemical Properties
Off-White Powder
Originator
Meiji Seika (Japan)
Uses
antidepressant
Uses
An antibacterial. Third generation cephalosporin
Uses
Cefditoren Pivoxil is an antibacterial and is a third generation cephalosporin.
Definition
ChEBI: The pivaloyloxymethyl ester prodrug of cefditoren.
Manufacturing Process
A mixture of THF (250 ml) and water (150 ml) was stirred under inert
atmosphere. At 0°-1°C, 7-amino-3-[(Z)-2-(methyl-5-thiazolyl)vinyl]-3-cephem-4-carboxylic acid (25.0 g) and 2-mercapto-5-phenyl-1,3,4-
oxadiazolyl-(Z)-2-(2-aminothiazol-4-yl)-2-methoxyimino acetate (33.3 g) were
added. Triethylamine (10.5 g) was slowly added to reaction by maintaining the
pH between 7.5 to 8.5. The reaction was monitored by HPLC. After 4-5 hrs.,
the reaction mixture was extracted by methylene chloride. The aqueous layer
is subjected for charcoal (0.125 g) treatment. Ethylacetate was added to the
filtrate and the solution was acidified with diluted HCl at 10°C to pH 3.0. The
solid separated was filtered, washed with water and ethylacetate and then
dried under vacuum at 40-45°C to get 3-[(Z)-2-(4-methyl-5-thiazolyl)vinyl]-7-
[(Z)-(2-aminothiazolyl-4-yl)-2-(methoxyimino)acetamido]-3-cephem-4-
carboxylic acid (Cefditoren acid), 35.0 g (yield 90%), HPLC (purity)=96-98%.
In practice it is often used as Cefditoren pivoxil.
brand name
Meiact
Therapeutic Function
Antibiotic
Antimicrobial activity
It exhibits good activity against staphylococci, streptococci (but not enterococci), H. influenzae and M. catarrhalis, including β-lactamase-producing strains. Isolates of Str. pneumoniae exhibiting reduced susceptibility to penicillin are less susceptible (MIC 0.125–2 mg/L). Most enterobacteria, including many Enterobacter, Citrobacter, Serratia and Proteus spp., are susceptible. It is not active against Ps. aeruginosa, Sten. maltophilia or atypical respiratory pathogens such as Chlamydophila pneumoniae and M. pneumoniae. It is stable to staphylococcal and common enterobacterial β-lactamases.
Pharmacokinetics
Oral absorption: c. 70%
Cmax 200 mg oral: c. 1.8 mg/L after 1.5–3 h
Plasma half-life: 0.8–1.3 h
Volume of distribution: 9.3 L
Plasma protein binding: 88%
After oral administration the pivaloyl ester is rapidly cleaved
by esterases in the gut wall. Ingestion with food improves
the bioavailability. Plasma concentrations are raised in
elderly patients. There is no accumulation on repeated
dosing.
It is excreted unchanged in the urine with a half-life of
around 1.5 h, achieving a concentration of 150–200 mg/L
within 4 h. Dosage adjustment is recommended in patients
with deteriorating renal function.
Clinical Use
It has been advocated for community-acquired upper and lower respiratory tract infections and skin infections.
Side effects
In common with other pivoxil esters it may cause carnitine deficiency. Other side effects are those common to cephalosporins, mainly gastrointestinal disturbance.
Cefditoren pivoxil Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29791 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 18747 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29884 | 58 |
| Cangzhou Wanyou New Material Technology Co.,Ltd | 18631714998 | sales@czwytech.com | CHINA | 904 | 58 |
| Shanghai Longyu Biotechnology Co., Ltd. | +8619521488211 | info@longyupharma.com | China | 2524 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49374 | 58 |
| Hebei Runbin Biotechnology Co. LTD | 13180553332 | 2179877681@qq.com | CHINA | 990 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 32079 | 58 |
Related articles
- The correlational research of Cefditoren pivoxil
- Cefditoren pivoxil is a third-generation oral cephalosporin with a broad spectrum of activity against pathogens, including bot....
- Aug 19,2019
View Lastest Price from Cefditoren pivoxil manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-14 | Cefditoren pivoxil
117467-28-4
|
US $54.00-143.00 / mg | 97.3% | 10g | TargetMol Chemicals Inc. | ||
 |
2024-11-14 | Cefditoren pivoxil
117467-28-4
|
US $54.00-143.00 / mg | 97.3% | 10g | TargetMol Chemicals Inc. | ||
 |
2024-04-10 | Cefditoren Pivoxil
117467-28-4
|
US $0.00-0.00 / mg | 10mg | 90%+ | 10g | Guangzhou PI PI BIOTECH INC |
-

- Cefditoren pivoxil
117467-28-4
- US $54.00-143.00 / mg
- 97.3%
- TargetMol Chemicals Inc.
-

- Cefditoren pivoxil
117467-28-4
- US $54.00-143.00 / mg
- 97.3%
- TargetMol Chemicals Inc.
-

- Cefditoren Pivoxil
117467-28-4
- US $0.00-0.00 / mg
- 90%+
- Guangzhou PI PI BIOTECH INC