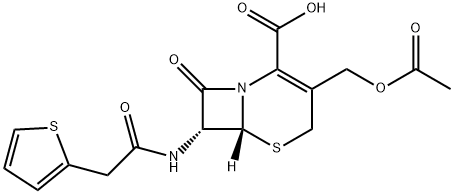
Cephalothin synthesis
- Product Name:Cephalothin
- CAS Number:153-61-7
- Molecular formula:C16H16N2O6S2
- Molecular Weight:396.44
Yield:153-61-7 154 g
Reaction Conditions:
in 1,2-dimethoxyethane at 15 - 25; for 1 h;Irradiation;Solvent;Temperature;Concentration;
Steps:
3 Example 3
To a 2000 mL three-necked flask was added 71 g (0.5 mol) of 2-thiopheneacetic acid,Organic solvent ethylene glycol dimethyl ether 600mL,Then add 80 mL of morpholine andTrifluoroacetic acid succinimidyl ester212 g (1.0 mol), and then the temperature was programmed at a temperature of 10 ° C to 15 ° C.4.5 hours, the reaction was completed, and the reaction solution containing the active ester was obtained by filtration,The reaction solution containing the active ester was slowly added dropwise to a prefabricated solution containing 136 g (0.5 mol) of 7-ACA in 1000 mL of ethylene glycol dimethyl ether,The temperature was irradiated at 15 ° C to 25 ° C for 1 hour,After the condensation reaction was completed, the reaction solution was added dropwise with 10% by weight hydrochloric acid solution, the pH was adjusted to 1.5 to 2.0, and then allowed to stand, the layers were collected, the organic phase was collected,The collected organic phase was rapidly added to 1000 mL of aqueous sodium bicarbonate solution, the pH was adjusted to 6.5 to 7.0,The temperature was then controlled at 20 ° C to 25 ° C, 10% by weight hydrochloric acid was slowly added, the pH of the system was adjusted to 1.5, stirred, the crystals were raised for 1 hour,The filtrate was filtered to give a wet product and dried at 40 & lt; 0 & gt; C154 g of product was obtained as a white solid, cefuroxime, in a yield of 96.9%.
References:
CN104610280,2017,B Location in patent:Paragraph 0020; 0021; 0022; 0023; 0024; 0025; 0026
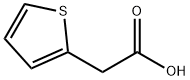
1918-77-0
410 suppliers
$5.00/100mg

957-68-6
437 suppliers
$14.00/5g

153-61-7
161 suppliers
$6.00/1g

39098-97-0
413 suppliers
$6.00/5g
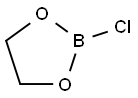
1192-03-6
3 suppliers
inquiry

957-68-6
437 suppliers
$14.00/5g

153-61-7
161 suppliers
$6.00/1g

