
CHEMBRDG-BB 4024637 synthesis
- Product Name:CHEMBRDG-BB 4024637
- CAS Number:905811-04-3
- Molecular formula:C13H11ClN2O
- Molecular Weight:246.69

6282-15-1
3 suppliers
inquiry

905811-04-3
9 suppliers
$46.00/1g
Yield:905811-04-3 83.4%
Reaction Conditions:
with iron(0);ammonia hydrochloride in methanol;lithium hydroxide monohydrate at 70; for 2 h;
Steps:
4.1.12. synthesis of N-(3-Aminophenyl)-4-chlorobenzamide (13)
To a solution of 12 (2.18 g, 7.9 mmol) and NH4Cl (4.22 g, 39.5mmol) in 32 mL of methanol and 8 mL of H2O was added Fe (2.21 g,79.0 mmol). The mixture was heated for 2 h at 70 C. After completion ofthe reaction as indicated by TLC, the mixture was filtered through aCelite bed. The filtrate was concentrated in vacuo, and the residue wasdiluted with water. The resulting solution was alkalized to pH 8-10 with2 N aqueous NaOH. The precipitate was filtrated, and the filter cake wasdried under vacuum to afford 13 as a brown solid in 83.4 % yield.
References:
Tian, Ye;Li, Shuo;Dong, Kuan;Su, Xiaolu;Fu, Siyu;Lv, Xuening;Duan, Meibo;Yang, Ting;Han, Yu;Hu, Guangda;Liu, Jialu;Sun, Yanping;Yue, Hao;Sun, Yongjun;Zhang, Huimin;Du, Zhidian;Miao, Zhenyu;Tong, Minghui;Liu, Yajing;Qin, Mingze;Gong, Ping;Hou, Yunlei;Gao, Zibin;Zhao, Yanfang [Bioorganic Chemistry,2022,vol. 127,art. no. 105898]
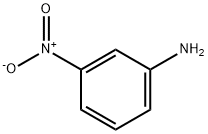
99-09-2
3 suppliers
$14.00/1g

905811-04-3
9 suppliers
$46.00/1g
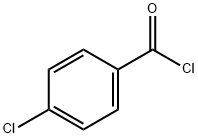
122-01-0
371 suppliers
$13.00/25g

905811-04-3
9 suppliers
$46.00/1g

108-45-2
444 suppliers
$13.00/25g

905811-04-3
9 suppliers
$46.00/1g
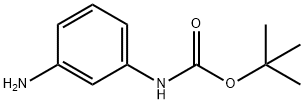
68621-88-5
193 suppliers
$6.00/1g

905811-04-3
9 suppliers
$46.00/1g