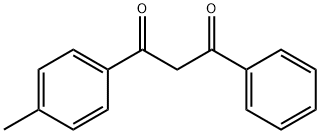
CHEMBRDG-BB 5213821 synthesis
- Product Name:CHEMBRDG-BB 5213821
- CAS Number:25855-99-6
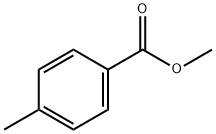
- Molecular formula:C16H14O2
- Molecular Weight:238.28
Yield:25855-99-6 98.8%
Reaction Conditions:
with sodium methylate in 5,5-dimethyl-1,3-cyclohexadiene; for 1.25 h;Inert atmosphere;Reflux;Microwave irradiation;Claisen Condensation;
Steps:
13 EXAMPLE 13Synthesis of Benzoyl p-MethylBenzoylMethane (BpMBM) Using the Process According to theInvention
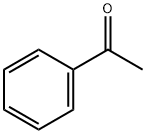
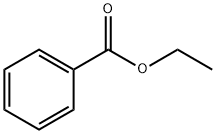
10159] The experimental apparatus consists of a classic glass, double jacketed chemical engineering reactor with a volume of 1 litre with an effective mixing system. This is topped with a separating column fitted with a variable-reflux condenser. It also has a recirculating loop fitted with a gear- type pump and a 600 W microwave generatot10160] Add 550 mL xylenes, 90.02 g fused methyl benzoate and 34.02 g powdered sodium methoxide. Once the reagents have been added, render the reactor inert with a continuous flow of nitrogen gas. Recirculate the mixture through the external circuit at a rate of 15 kg/h. Bring to boiling and total reflux, and then switch the microwave sourceon.10161] Add 68.42 g acetophenone over one hout Once it has all been added, let the reaction continue for another 15 minutes. Throughout all this time, draw the methanol off the reaction mixture. After the 15 minutes of finishing time, switch off the microwave source and the heatet AcidiFy themixture and then wash it. 10162] Analysis of the organic phase by gas phase chromatography shows that almost all the acetophenone is consumed and that the BpMBM titre is 98.8%.10163] BpMBM productivity during the reaction phase is112.9 kg/hIm3.
References:
US2014/88325,2014,A1 Location in patent:Paragraph 0159; 0160; 0161; 0162; 0163

20442-65-3
9 suppliers
inquiry

25855-99-6
10 suppliers
$75.00/1g
93-89-0
503 suppliers
$13.00/100g
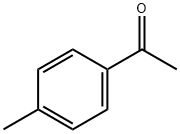
122-00-9
681 suppliers
$5.00/25g

25855-99-6
10 suppliers
$75.00/1g
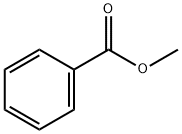
93-58-3
470 suppliers
$14.00/25mL

122-00-9
681 suppliers
$5.00/25g

25855-99-6
10 suppliers
$75.00/1g

20442-65-3
9 suppliers
inquiry
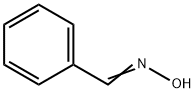
932-90-1
127 suppliers
$17.67/1gm:

25855-99-6
10 suppliers
$75.00/1g
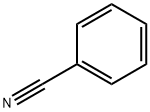
100-47-0
359 suppliers
$14.14/25gm: