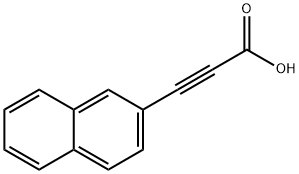
CHEMBRDG-BB 5217434 synthesis
- Product Name:CHEMBRDG-BB 5217434
- CAS Number:4843-43-0
- Molecular formula:C13H8O2
- Molecular Weight:196.2
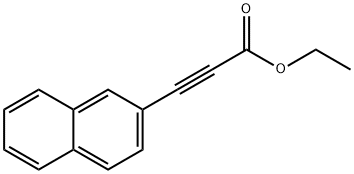
445491-33-8
2 suppliers
inquiry

4843-43-0
18 suppliers
$65.00/100mg
Yield:4843-43-0 109 mg
Reaction Conditions:
with lithium hydroxide monohydrate in tetrahydrofuran;methanol;water at 40; for 3 h;
Steps:
6.1.12. General procedure for preparing propiolic acid derivatives(GP-3)
General procedure: To a stirred solution of carbon tetrabromide (1.5 eq.) and triphenylphosphine (3.0 eq) in dehydrated dichloromethane at ambient temperature was added an appropriate aldehyde (1.0 eq.). The reaction mixture was stirred at ambient temperature overnight, then poured into water, and the whole was extracted with chloroform. The extract was washed with brine, dried over anhydrous magnesium sulfate and concentrated. The residue was purified by silica gel column chromatography (eluent; n-hexane: ethylacetate 10:1 v/v) to afford the dibromo alkene derivative. To a stirred solution of this intermediate in dehydrated tetrahydrofuran was slowly added n-butyl lithium (2.5 eq.) via a syringe at 0 °C. The reaction mixture was stirred for 0.5 h at 0° C and then ethylchloroformate (1.5 eq.) was slowly added. The reaction mixture was stirred for 2.0 h at 0 °C, then quenched with saturated aqueous ammonium chloride, and the whole was extracted with ethyl acetate. The extract was washed with brine, dried over anhydrous magnesium sulfate and concentrated. The residue was purified by silica gel column chromatography (eluent; n-hexane: ethyl acetate 10:1 v/v) to afford the ethyl acetylene carboxylate derivative. To a solution of this intermediate (1.0 eq.) in tetrahydrofuran/methanol/H2O was added lithium hydroxide monohydrate (3.0 eq.). The reaction mixture was stirred at 40 °C for 3 h. The resulting mixture was concentrated and acidified with 1M HCl, and the whole was extracted with ethyl acetate. The extract was washed with brine, dried over anhydrous magnesium sulfate and concentrated to afford the title compound.
References:
Ohashi, Masao;Gamo, Kanae;Tanaka, Yuta;Waki, Minoru;Beniyama, Yoko;Matsuno, Kenji;Wada, Jun;Tenta, Masafumi;Eguchi, Jun;Makishima, Makoto;Matsuura, Nobuyasu;Oyama, Takuji;Miyachi, Hiroyuki [European Journal of Medicinal Chemistry,2015,vol. 90,p. 53 - 67]

124-38-9
129 suppliers
$214.00/14L
2949-26-0
108 suppliers
$24.00/250mg

4843-43-0
18 suppliers
$65.00/100mg
580-13-2
504 suppliers
$6.00/1g

471-25-0
233 suppliers
$10.00/1g

4843-43-0
18 suppliers
$65.00/100mg
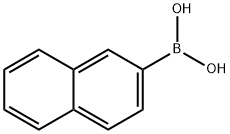
32316-92-0
477 suppliers
$6.00/1g
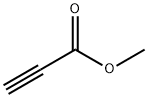
922-67-8
348 suppliers
$6.00/1g

4843-43-0
18 suppliers
$65.00/100mg
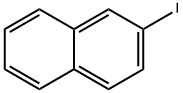
612-55-5
111 suppliers
$48.00/500mg

471-25-0
233 suppliers
$10.00/1g

4843-43-0
18 suppliers
$65.00/100mg