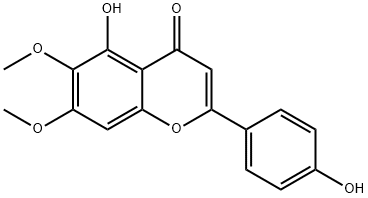
cirsimaritin synthesis
- Product Name:cirsimaritin
- CAS Number:6601-62-3
- Molecular formula:C17H14O6
- Molecular Weight:314.29
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

6601-62-3
88 suppliers
$100.00/0.5 mg
Yield: 91%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in ethanol under 760.051 Torr; for 12 h;
Steps:
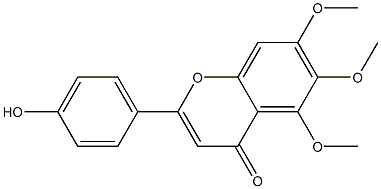
19 5-Hydroxy-2-(4-hydroxyphenyl)-6,7-dimethoxy-4Hchromen-4-one (7)
4.1.19
5-Hydroxy-2-(4-hydroxyphenyl)-6,7-dimethoxy-4H-chromen-4-one (7)
To a solution of 23 (100 mg, 0.25 mmol) dissolved in ethanol (30 ml) was added 10% Pd/C (10 mg) with vigorous stirring.
The reaction vessel was then evacuated and the atmosphere replaced with hydrogen.
After 12 h, the reaction mixture was filtered and the filtrate concentrated, the crude material was purified by column chromatography (20% ethyl acetate in petroleum ether) to afford 7 (71 mg, 91% yield) as a yellow solid. 1H NMR (300 MHz, DMSO-d6) δ 3.92 (s, 3H, -CH3), 3.98 (s, 3H, -CH3), 6.86 (s, 1H, 3-H), 7.52 (s, 1H, 8-H), 7.36 (d, 2H, J = 8.7 Hz, 3',5'-H), 8.15 (d, 2H, J = 8.7 Hz, 2',6'-H), 10.40 (s, 1H, 4'-OH), 13.05 (s, 1H, 5-OH); ESI-MS: m/z 313 [M - H]-.
References:
Shi, Zhi-Hao;Li, Nian-Guang;Wang, Zhen-Jiang;Tang, Yu-Ping;Dong, Ze-Xi;Zhang, Wei;Zhang, Peng-Xuan;Gu, Ting;Wu, Wen-Yu;Yang, Jian-Ping;Duan, Jin-Ao [European Journal of Medicinal Chemistry,2015,vol. 106,p. 95 - 105]
6938-18-7
1 suppliers
inquiry

6601-62-3
88 suppliers
$100.00/0.5 mg

1447-88-7
174 suppliers
$25.00/1mg

74-88-4
343 suppliers
$15.00/10g

6601-62-3
88 suppliers
$100.00/0.5 mg
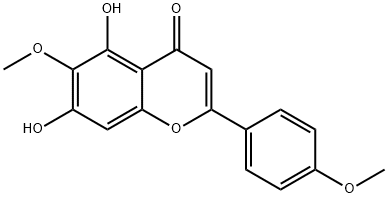
520-12-7
138 suppliers
$62.00/5mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

6601-62-3
88 suppliers
$100.00/0.5 mg
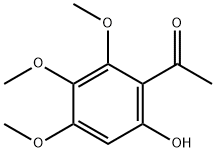
22248-14-2
24 suppliers
inquiry

6601-62-3
88 suppliers
$100.00/0.5 mg