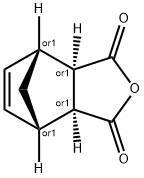
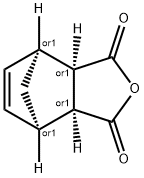
CIS-5-NORBORNENE-EXO-2,3-DICARBOXYLIC ANHYDRIDE synthesis
- Product Name:CIS-5-NORBORNENE-EXO-2,3-DICARBOXYLIC ANHYDRIDE
- CAS Number:2746-19-2
- Molecular formula:C9H8O3
- Molecular Weight:164.16
Yield:2746-19-2 82.7%
Reaction Conditions:
in hexane;ethyl acetate at 60; for 1 h;Cooling with ice;Diels-Alder Cycloaddition;
Steps:
1.2 (2) Preparation of cis-5-norbornene-endo-5,6-dicarboxylic acid anhydride
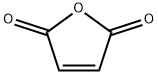
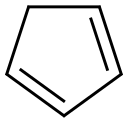
In a 1 L flask, 260 g (2.65 mol) of maleic anhydride and 500 mL of ethyl acetate were stirred, and then 200 mL of hexane was added to dissolve it completely. The flask was charged with 175 g (2.65 mol) of the cyclopentadiene prepared in the above (1) in a state of lowering the temperature with an ice bath. After the whole amount was added, the ice bath was removed, and the reaction was conducted at 60 ° C for 1 hour. After completion of the reaction, the reaction product was allowed to stand at -20 for 8 hours to effect recrystallization. The obtained solid was filtered and dried to obtain 360 g of a white compound represented by the formula (3) (yield: 82.7%). The obtained compounds were identified by NMR (1H and 13C) (JEOL, JNM-LA400) and IR (AVATAR, 360 FT-IR), and the results are shown in FIGS. 2 and 3, respectively.
References:
Kolon Industries INC.;Park Su-yeon;Park Won-jin;Park Gi-hyeon;Park Jun-hyo KR2018/65404, 2018, A Location in patent:Paragraph 0092; 0097; 0098
129-64-6
236 suppliers
$7.00/25g

2746-19-2
268 suppliers
$6.00/5g
108-31-6
838 suppliers
$13.47/50 G
542-92-7
90 suppliers
inquiry

129-64-6
236 suppliers
$7.00/25g

2746-19-2
268 suppliers
$6.00/5g
![Cis-exo-bicyclo[2.2.1]hept-5-ene-2,3-dicarboxylic acid](/CAS/GIF/21196-51-0.gif)
21196-51-0
7 suppliers
inquiry

2746-19-2
268 suppliers
$6.00/5g
110-17-8
1021 suppliers
$9.00/5g

129-64-6
236 suppliers
$7.00/25g

108-31-6
838 suppliers
$13.47/50 G

2746-19-2
268 suppliers
$6.00/5g