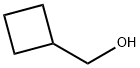
Cyclobutylmethyl 4-methylbenzenesulfonate synthesis
- Product Name:Cyclobutylmethyl 4-methylbenzenesulfonate
- CAS Number:13295-53-9
- Molecular formula:C12H16O3S
- Molecular Weight:240.32
Yield:13295-53-9 97%
Reaction Conditions:
with 4-dimethylaminopyridine in dichloromethane at 0 - 25;
Steps:
I.1
A solution of cyclobutanemethanol (4.0 g, 46.4 mmol) in dichloromethane (28 mL) at 25° C. was treated with 4-dimethylaminopyridine (6.23 g, 50.9 mmol). The reaction was then cooled to 0° C. and was treated with para-toluenesulfonylchloride (8.95 g, 46.94 mmol). The reaction was allowed to slowly warm to 25° C. and was allowed to stir overnight. After this time, the reaction was partitioned between water (200 mL) and methylene chloride (2×200 mL). The combined organics were washed with a 1N aqueous hydrochloric acid solution and a saturated aqueous sodium chloride solution (1×200 mL), dried over magnesium sulfate, filtered and concentrated in vacuo to afford toluene-4-sulfonic acid cyclobutylmethyl ester (10.87 g, 97%) as colorless oil which was used without further purification.
References:
US2009/264434,2009,A1 Location in patent:Page/Page column 45-46
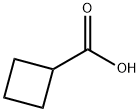
3721-95-7
413 suppliers
$7.00/5g

13295-53-9
17 suppliers
$45.00/10mg

3779-29-1
260 suppliers
$19.00/10g

13295-53-9
17 suppliers
$45.00/10mg
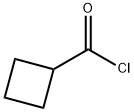
5006-22-4
182 suppliers
$12.00/1g

13295-53-9
17 suppliers
$45.00/10mg

14924-53-9
162 suppliers
$7.00/5g

13295-53-9
17 suppliers
$45.00/10mg