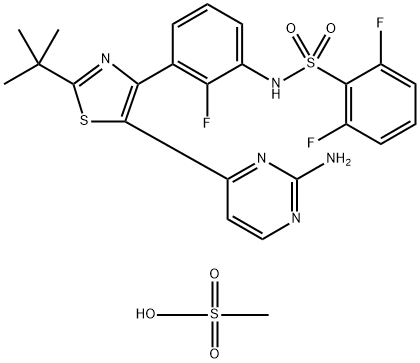
Dabrafenib Mesylate synthesis
- Product Name:Dabrafenib Mesylate
- CAS Number:1195768-06-9
- Molecular formula:C24H24F3N5O5S3
- Molecular Weight:615.66
75-75-2
608 suppliers
$10.00/5g
1195765-45-7
262 suppliers
$9.00/1mg

1195768-06-9
175 suppliers
inquiry
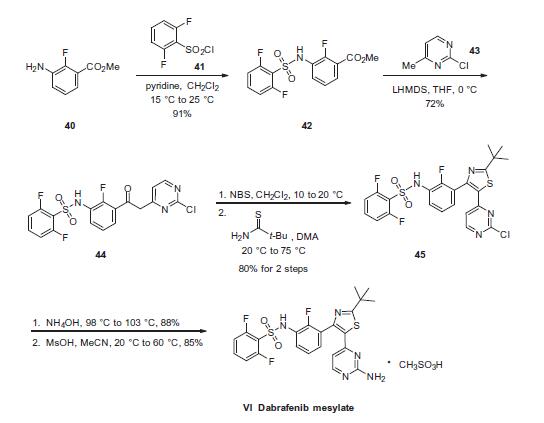
Yield:1195768-06-9 87%
Reaction Conditions:
in isopropyl alcohol at 20; for 3 h;
Steps:
1 The Preparation of the Known Crystal Form I
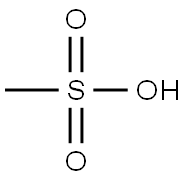
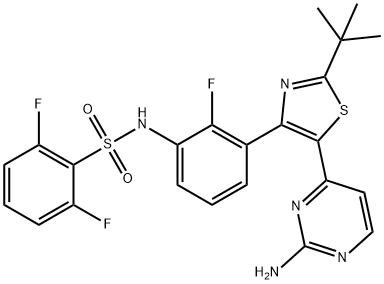
Add methylsulfonic acid (0.131 ml, 0.393 mmol) into the isopropanol solution of N-{3-[5-(2-amino-4-pyrimidinyl)-2-(1,1-dimethylethyl)-1,3-thiazol-4-yl]-2-fluorophenyl}-2,6-difluorobenzenesulfonamide (204 mg, 0.393 mmol) and stir the solution at room temperature for 3 h to obtain a white precipitate, filter the slurry and wash it with ethyl ether to obtain N-[3-[5-(2-amino-4-pyrimidinyl)-2-(1,1-dimethylethyl)-1,3-thiazo-4-yl]-2-fluorophenyl]-2,6-difluorobenzenesulfonamide methanesulfonate, which is a white crystals (221 mg, 87% yield).
1HNMR (400 MHz, DMSO-d6) δ ppm 10.85 (s, 1H) 7.92-8.05 (m, 1H), 7.56-7.72 (m, 1H), 6.91-7.50 (m, 7H), 5.83-5.98 (m, 1H), 2.18-2.32 (m, 3H), 1.36 (s, 9H). j0114] The X-ray powder diffraction pattern of the obtained crystals as shown in FIG. 10 is consistent with that mentioned in the patent documents W02009/137391 or CN200980126781 .6.10115] The DSC thermogram is shown in FIG. 11: the melting range of the Known Crystal Form I is 247° C.250°C.10116] The TGA thermogram is shown in FIG. 12: the decomposition temperature is 261° C.
References:
Hangzhou Pushai Pharmaceutical Technology Co., LTD.;LAO, Haiping;SHENG, Xiaoxia;Sheng, Xiaohong US2016/46615, 2016, A1 Location in patent:Paragraph 0113; 0114; 0115; 0116
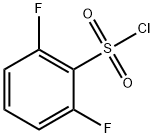
60230-36-6
237 suppliers
$6.00/1g

1195768-06-9
175 suppliers
inquiry

1195768-20-7
48 suppliers
inquiry

1195768-06-9
175 suppliers
inquiry
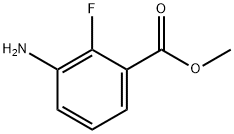
1195768-18-3
218 suppliers
$11.00/100mg

1195768-06-9
175 suppliers
inquiry

1195768-19-4
81 suppliers
$123.00/250mg

1195768-06-9
175 suppliers
inquiry