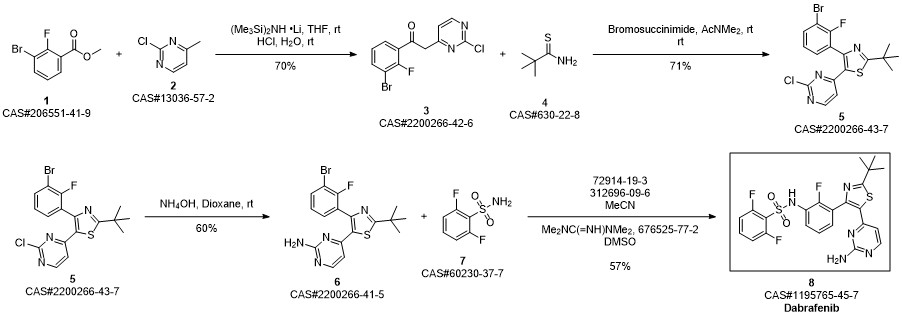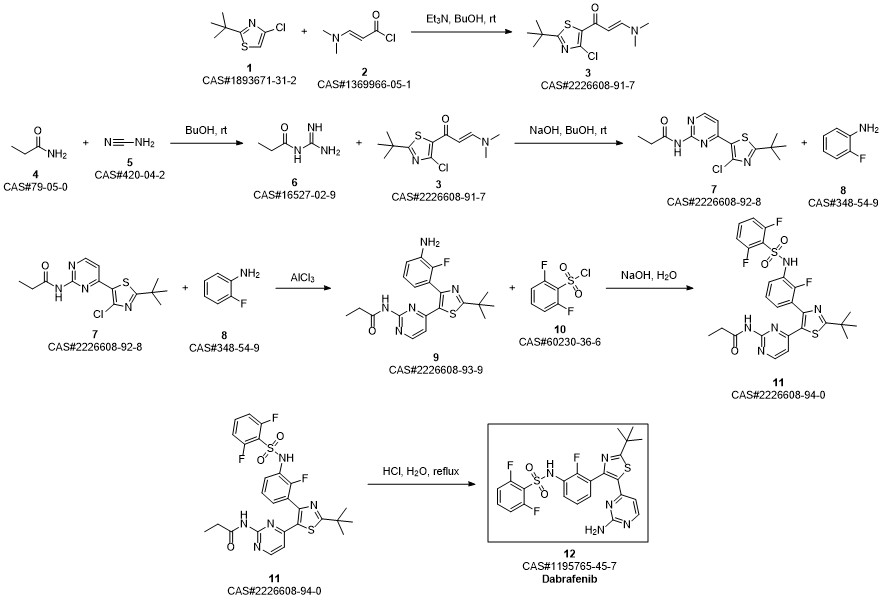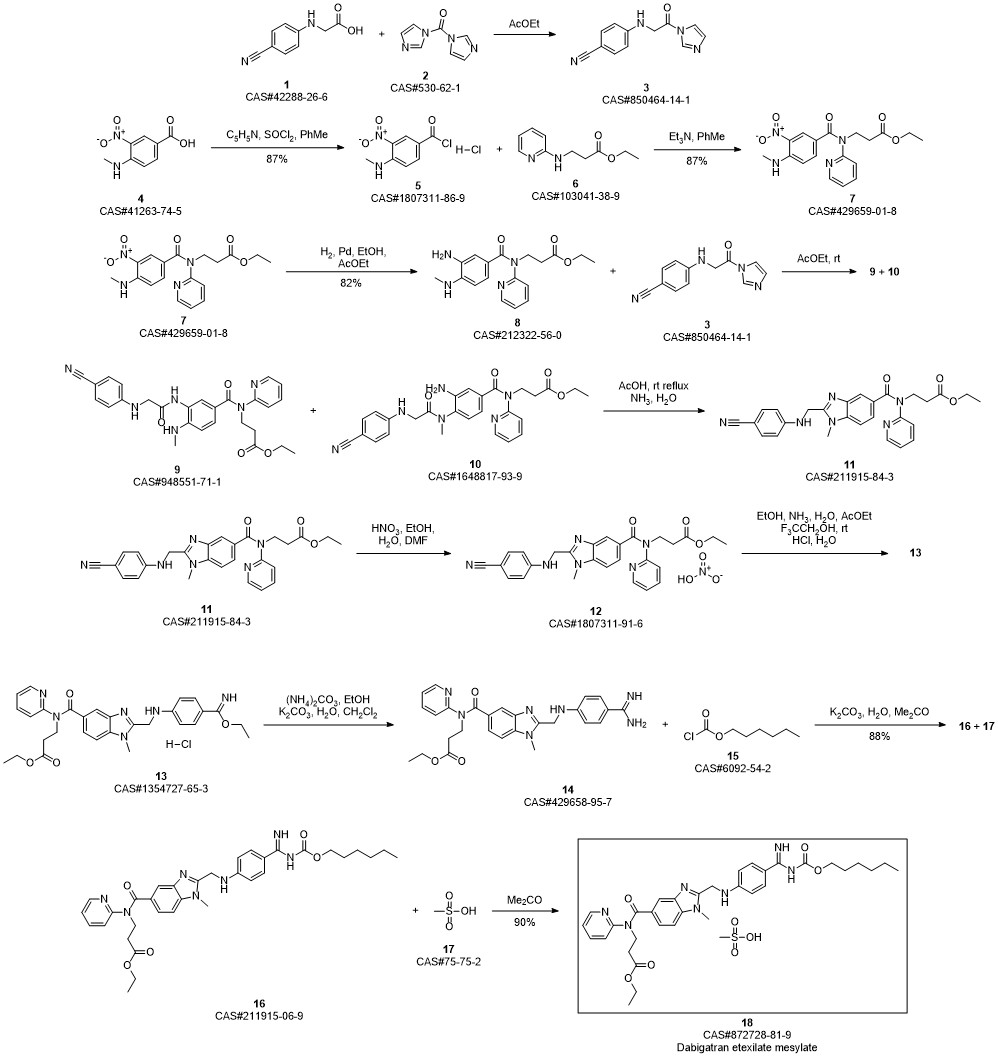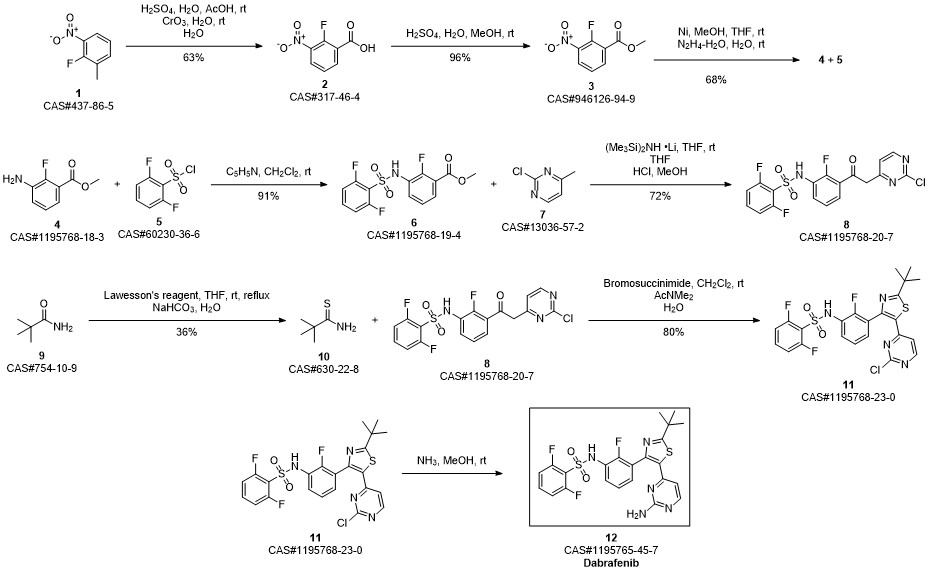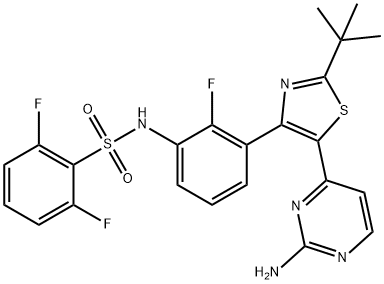
Dabrafenib synthesis
- Product Name:Dabrafenib
- CAS Number:1195765-45-7
- Molecular formula:C23H20F3N5O2S2
- Molecular Weight:519.56
Rheault, Tara R.; Stellwagen, John C.; Adjabeng, George M.; Hornberger, Keith R.; Petrov, Kimberly G.; Waterson, Alex G.; Dickerson, Scott H.; Mook, Robert A.; Laquerre, Sylvie G.; King, Alastair J.; Rossanese, Olivia W.; Arnone, Marc R.; Smitheman, Kimberly N.; Kane-Carson, Laurie S.; Han, Chao; Moorthy, Ganesh S.; Moss, Katherine G.; Uehling, David E. Discovery of Dabrafenib: A Selective Inhibitor of Raf Kinases with Antitumor Activity against B-Raf-Driven Tumors. ACS Medicinal Chemistry Letters. Volume 4. Issue 3. Pages 358-362. Journal; Online Computer File. (2013).
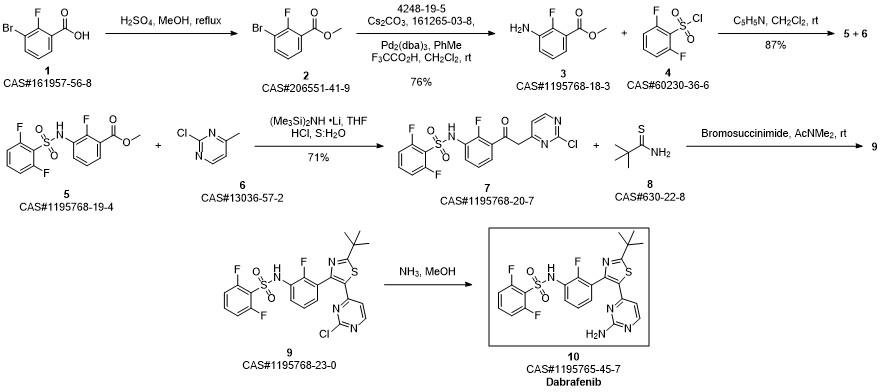
![N-{3-[5-(2-chloro-4-pyriMidinyl)-2-(1,1-diethylethyl)-1,3-thiazol-4-yl]-2-fluoraphenyl}-2,6-difluorobenzenesulfonaMide](/CAS/20150408/GIF/1195768-23-0.gif)
1195768-23-0
68 suppliers
inquiry

1195765-45-7
262 suppliers
$9.00/1mg
Yield:1195765-45-7 88%
Reaction Conditions:
with ammonium hydroxide in water at 20 - 103;Product distribution / selectivity;sealed tube;
Steps:
3.D
Step D: A/-{3-[5-(2-amino-4-pyhmidinyl)-2-(1 ,1 -dimethylethyl)-1 ,3-thiazol-4-yl]-2- fluorophenyl}-2,6-difluorobenzenesulfonannideIn 1 gal pressure reactor, a mixture of A/-{3-[5-(2-chloro-4-pyrinnidinyl)-2-(1 ,1 - dimethylethyl)-1 ,3-thiazol-4-yl]-2-fluorophenyl}-2,6-difluorobenzenesulfonamide (120 g) prepared in accordance with Step C, above, and ammonium hydroxide (28-30%, 2.4 L, 20 vol) was heated in the sealed pressure reactor to 98-103 °C and stirred at this temperature for 2 hours. The reaction was cooled slowly to room temperature (20 °C) and stirred overnight. The solids were filtered and washed with minimum amount of the mother liquor and dried under vacuum. The solids were added to a mixture of EtOAc (15 vol)/ water (2 vol) and heated to complete dissolution at 60-70 °C and the aqueous layer was removed and discarded. The EtOAC layer was charged with water (1 vol) and neutralized with aq. HCI to ~pH 5.4-5.5. and added water (1 vol). The aqueous layer was removed and discarded at 60-70 °C. The organic layer was washed with water (1 vol) at 60-70 °C and the aqueous layer was removed and discarded. The organic layer was filtered at 60 °C and concentrated to 3 volumes. EtOAc (6 vol) was charged into the mixture and heated and stirred at 72 °C for 10 min , then cooled to 20°C and stirred overnight. EtOAc was removed via vacuum distillation to concentrate the reaction mixture to ~3 volumes. The reaction mixture was maintained at ~65-70°C for ~30mins. Product crystals having the same crystal form as those prepared in Example 58b (and preparable by the procedure of Example 58b), above, in heptanes slurry were charged. Heptane (9 vol) was slowly added at 65-70 °C. The slurry was stirred at 65-70 °C for 2- 3 hours and then cooled slowly to 0-5°C. The product was filtered, washed withEtOAc/heptane (3/1 v/v, 4 vol) and dried at 45°C under vacuum to obtain A/-{3-[5-(2- amino-4-pyrimidinyl)-2-(1 ,1 -dimethylethyl)-1 ,3-thiazol-4-yl]-2-fluorophenyl}-2,6- difluorobenzenesulfonamide (102.3 g, 88%).
References:
GLAXOSMITHKLINE LLC;DUMBLE, Melissa;KUMAR, Rakesh;LAQUERRE, Sylvie;LEBOWITZ, Peter WO2011/47238, 2011, A1 Location in patent:Page/Page column 15-16
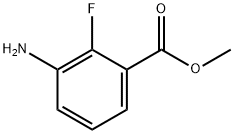
1195768-18-3
218 suppliers
$11.00/100mg

1195765-45-7
262 suppliers
$9.00/1mg

1195768-19-4
81 suppliers
$123.00/250mg

1195765-45-7
262 suppliers
$9.00/1mg

1195768-20-7
48 suppliers
inquiry

1195765-45-7
262 suppliers
$9.00/1mg
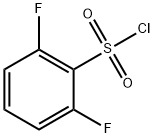
60230-36-6
237 suppliers
$6.00/1g

1195765-45-7
262 suppliers
$9.00/1mg