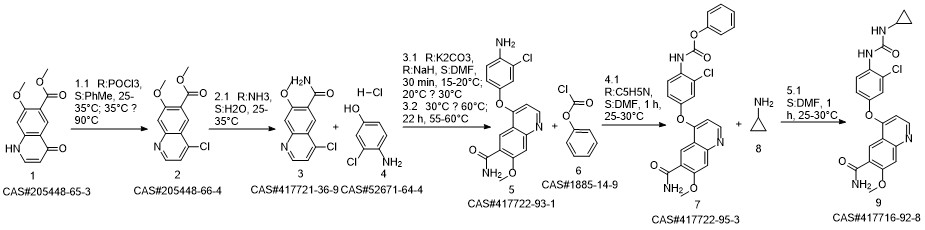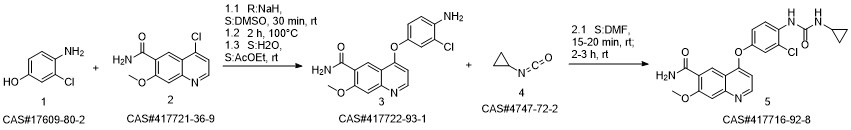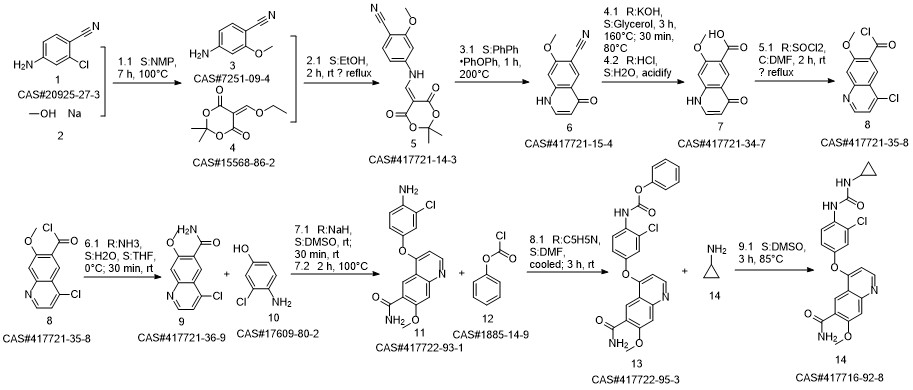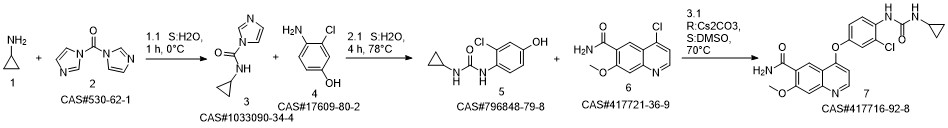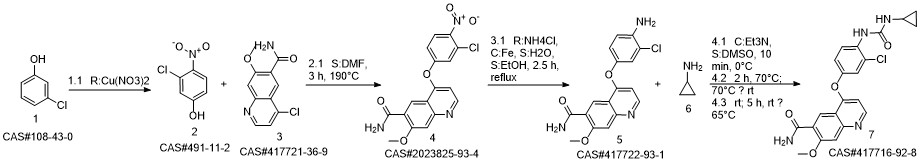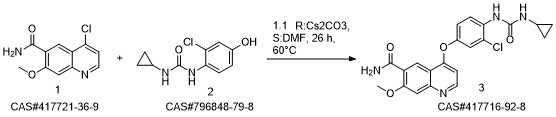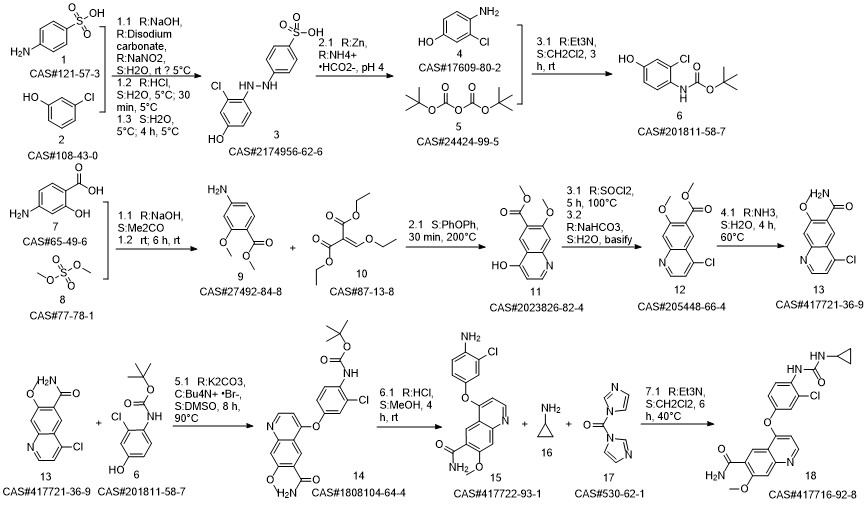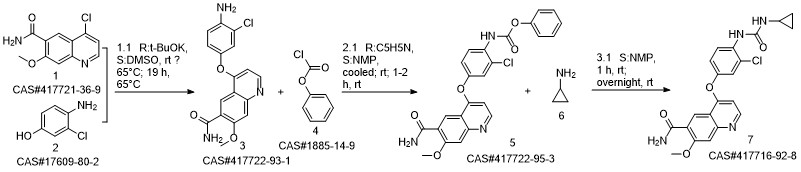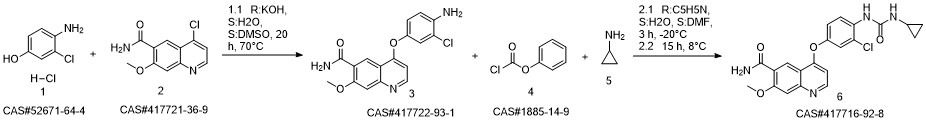
Lenvatinib synthesis
- Product Name:Lenvatinib
- CAS Number:417716-92-8
- Molecular formula:C21H19ClN4O4
- Molecular Weight:426.85
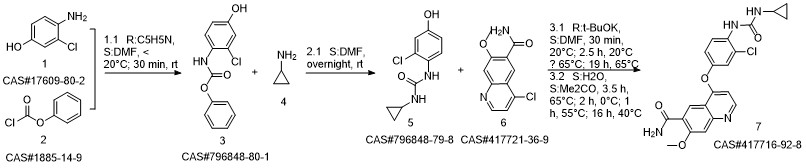
Reference: Naito, Toshihiko; Yoshizawa, Kazuhiro. Preparation of urea moiety-containing quinolinecarboxamide derivatives. WO 2005044788. (Assignee Eisai Co., Ltd., Japan)
417722-93-1
97 suppliers
inquiry
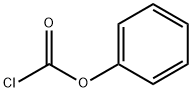
1885-14-9
335 suppliers
$10.00/1g
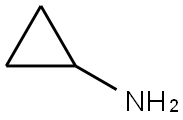
765-30-0
489 suppliers
$10.00/25g

417716-92-8
455 suppliers
$5.00/10mg
Yield:417716-92-8 90%
Reaction Conditions:
Stage #1: 4?(4?amino?3?chlorophenoxy)?7?methoxyquinoline?6?carboxamide;phenyl chloroformatewith pyridine in water;N,N-dimethyl-formamide at -20; for 3 h;Inert atmosphere;
Stage #2: Cyclopropylamine in water;N,N-dimethyl-formamide at 8; for 15 h;Inert atmosphere;Time;
Steps:
2 Example 2 4-[3-Chloro-4-(cyclopropylaminocarbonyl)aminophenoxy]-7-methoxy-6-quinoline-carboxamide
Example 2 4-[3-Chloro-4-(cyclopropylaminocarbonyl)aminophenoxy]-7-methoxy-6-quinoline-carboxamide (0148) (0149) To a mixture of 26.0 kg of 4-(4-amino-3-chlorophenoxy)-7-methoxy-quinoline-6-carboxamide, 13.2 kg of pyridine, 1.36 kg of water and 196.0 L of N,N-dimethylformamide there was added 26.6 kg of phenyl chloroformate at -20° C. under a nitrogen atmosphere, and the mixture was stirred for 3 hours. Next, 19.4 kg of cyclopropylamine was further added at 8° C. and the mixture was stirred for 15 hours. After adding 13.0 L of water and 261.0 L of acetone to the reaction mixture, the deposited precipitate was filtered. The precipitate was rinsed with acetone, and the obtained solid was dried under reduced pressure to obtain 28.7 kg of a crude product of the title compound (89% yield). This was crystallized from 359.6 L of 1,3-dimethyl-2-imidazolidinone and 575.0 L of 2-propanol, to obtain 25.7 kg of compound (IV) (90% yield).
References:
US2017/233344,2017,A1 Location in patent:Paragraph 0148; 0149

417722-93-1
97 suppliers
inquiry
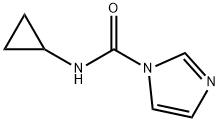
1033090-34-4
40 suppliers
$60.00/100mg

417716-92-8
455 suppliers
$5.00/10mg

417722-93-1
97 suppliers
inquiry
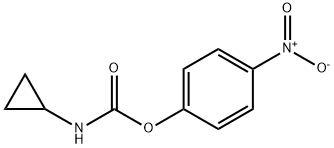
444288-42-0
2 suppliers
inquiry

417716-92-8
455 suppliers
$5.00/10mg
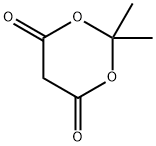
2033-24-1
638 suppliers
$6.00/25g
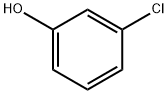
108-43-0
296 suppliers
$13.00/5g
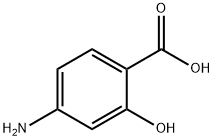
65-49-6
469 suppliers
$6.00/25g

1885-14-9
335 suppliers
$10.00/1g
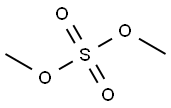
77-78-1
294 suppliers
$22.00/25g

765-30-0
489 suppliers
$10.00/25g

417716-92-8
455 suppliers
$5.00/10mg