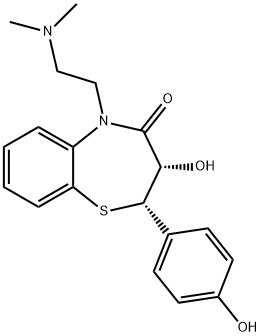
Deacetyl-O-demethyldiltiazem synthesis
- Product Name:Deacetyl-O-demethyldiltiazem
- CAS Number:84903-82-2
- Molecular formula:C19H22N2O3S
- Molecular Weight:358.45
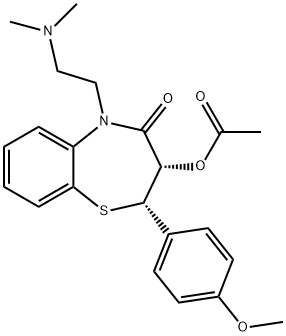
42399-41-7
141 suppliers
inquiry
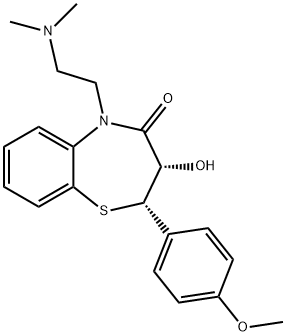
![1,5-Benzothiazepin-4(5H)-one, 5-[2-(dimethylamino)ethyl]-2,3-dihydro-3-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-, (2S,3S)-](/CAS/20210305/GIF/1268382-39-3.gif)
1268382-39-3
0 suppliers
inquiry
42399-40-6
61 suppliers
$50.00/2mg

84903-82-2
18 suppliers
inquiry
Yield:42399-40-6 1.75% ,1268382-39-3 1.8% ,84903-82-2 0.81%
Reaction Conditions:
Stage #1: diltiazemwith ethylenediaminetetraacetic acid trisodium salt;oxygen;manganese (II) acetate tetrahydrate;ascorbic acid in water; pH=4;Udenfriend reaction;
Stage #2: with ferrous(II) sulfate heptahydrate;ethylenediaminetetraacetic acid trisodium salt;oxygen in water at 45; for 2 h;Udenfriend reaction;
Steps:
12 Representative procedure-oxidation of buspirone
General procedure: Buspirone (822 mg, 2.13 mmol), EDTA trisodium salt (382 mg, 1.1 mmol), Mn(II)-acetate tetrahydrate (784 mg, 3.2 mmol) and ascorbic acid (2.63 g, 14.9 mmol) were dissolved in water (10.0 ml) and acetate buffer 2 M, pH 4 (12.0 ml) to give a colourless solution. A second solution containing EDTA trisodium salt (382 mg, 1.1 mmol) and iron(II) sulfate heptahydrate (593 mg, 2.13 mmol) in water (10.0 ml) was added to the mixture to give a brown solution. The mixture was well-stirred and heated to 45 ° C. Oxygen was bubbled into the mixture through a glass frit for 2 h. The reaction mixture was then adjusted to pH 8.5 using concd NaOH, and extracted with ethyl acetate. The combined organic layers were dried over Na2SO4. Solvents were removed under reduced pressure, and oxidation product 4 (44.6 mg, 5.16%) was isolated from the residue by automated, preparative HPLC (Phenomenex Gemini C18 column 75 * 20 mm, solvent gradient 20-98% MeOH in 0.1% Et3N (aq) over 13.0 min, flow rate 40 ml/min, UV detection [? = 254 nm]). 1H NMR (DMSO-d6): ? = 1.39-1.43 (8H, m), 1.61-1.63 (4H, m), 2.27 (2H, t), 2.35-2.37 (4H, m), 2.61 (4H, s), 3.52-3.56 (4H, m), 3.64 (2H, t), 8.00 (2H, s), 9.25 (1H, br s).
References:
Slavik, Roger;Peters, Jens-Uwe;Giger, Rudolf;Bürkler, Markus;Bald, Eric [Tetrahedron Letters,2011,vol. 52,# 7,p. 749 - 752] Location in patent:experimental part