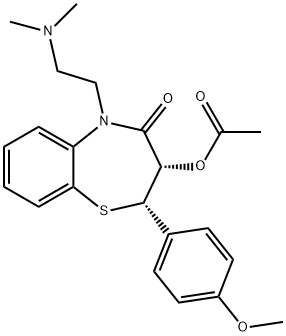
Diltiazem synthesis
- Product Name:Diltiazem
- CAS Number:42399-41-7
- Molecular formula:C22H26N2O4S
- Molecular Weight:414.52
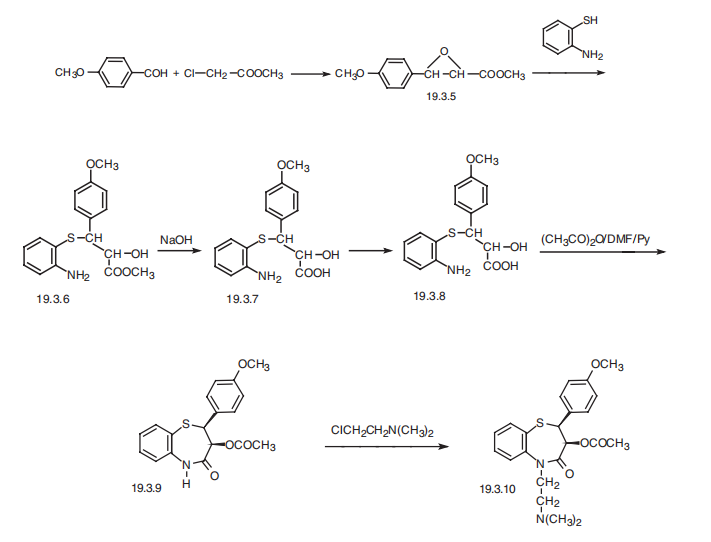

24393-56-4
212 suppliers
$5.00/250mg

42399-41-7
142 suppliers
inquiry
Yield:-
Steps:
Multi-step reaction with 6 steps
1: 85 percent / K3
2: 98 percent / SOCl2, pyridine / 2 h / 0 °C
3: 60 percent / toluene / 4 h / Heating
4: PTS / toluene / Heating
5: K2CO3, H2O / ethyl acetate / 12 h / Heating
6: Et3N / CH2Cl2
References:
Lohray;Jayachandran;Bhushan;Nandanan;Ravindranathan [Journal of Organic Chemistry,1995,vol. 60,# 18,p. 5983 - 5985]

33286-22-5
496 suppliers
$16.00/250mg

42399-41-7
142 suppliers
inquiry
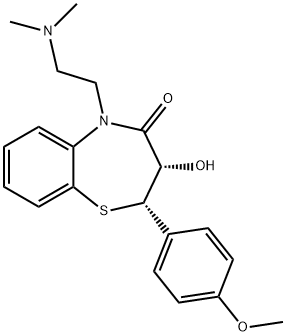
42399-40-6
60 suppliers
$50.00/2 mg

108-24-7
5 suppliers
$14.00/250ML

42399-41-7
142 suppliers
inquiry
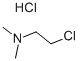
4584-46-7
5 suppliers
$15.50/5g
![(αS,βS)-β-[(2-AMinophenyl)thio]-α-hydroxy-4-Methoxybenzenepropanoic Acid Methyl Ester](/CAS/GIF/99109-07-6.gif)
99109-07-6
8 suppliers
$165.00/10mg

42399-41-7
142 suppliers
inquiry
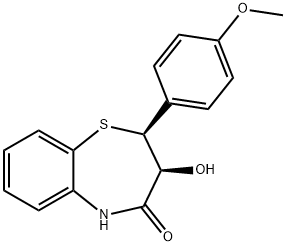
42399-49-5
173 suppliers
$6.00/5g

42399-41-7
142 suppliers
inquiry