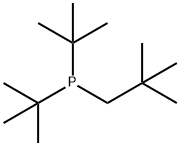
DI-T-BUTYLNEOPENTYLPHOSPHINE, MIN. 95 synthesis
- Product Name:DI-T-BUTYLNEOPENTYLPHOSPHINE, MIN. 95
- CAS Number:60633-21-8
- Molecular formula:C13H29P
- Molecular Weight:216.34
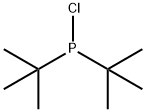
13716-10-4
260 suppliers
$22.00/1 g
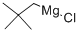
13132-23-5
32 suppliers
$334.00/50mL

60633-21-8
30 suppliers
$112.00/1g
Yield: 73%
Reaction Conditions:
Stage #1:di(tert-butyl)chlorophosphine;2,2-dimethylpropylmagnesium chloride in tetrahydrofuran;diethyl ether for 72 h;Heating / reflux;
Stage #2: with water;ammonium chloride in tetrahydrofuran;diethyl ether at 20;
Steps:
2 Example 2
Example 2 This example illustrates the preparation of di-tert-butyl-(2,2-dimethyl-propyl)-phosphine from di-tert-butyl-chlorophosphine. 28.80 g (0.160 mol) of di-t-butyl-chlorophosphine, 0.2 mole of neopentylmagnesium chloride in diethyl ether and 150 ml of THF) were refluxed under argon for 3 days. The reaction mixture was allowed to cool off to RT and an aqueous solution of ammonium chloride was added slowly. The organic phase was separated, and dried with magnesium sulfate. After removal of the solvent, the product was purified by distillation in vacuum. The yield of di-tert-butyl-(2,2-dimethyl-propyl)-phosphine was 25.26 g (73%) with b.p. 43-47° C./0.1 mm. 31-P-NMR (CD2Cl2)+19.76 ppm. 1H NMR (CD2Cl2) 1.17 (s, 9H, Me3C), 1.19 (s, 9H, Me3C), 2.47 (d, 2JPH=3.12 Hz, P-CH2-CMe3). Anal. Calcd. for C13H29P: C, 72.17; H, 13.51; P, 14.32. Found: C, 72.01; H, 13.49; P, 14.08.
References:
Ionkin, Alex Sergey US2006/281923, 2006, A1 Location in patent:Page/Page column 4