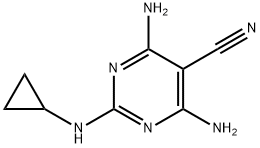
Dicyclanil synthesis
- Product Name:Dicyclanil
- CAS Number:112636-83-6
- Molecular formula:C8H10N6
- Molecular Weight:190.21
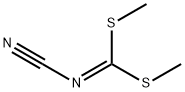
10191-60-3
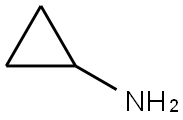
765-30-0

109-77-3

112636-83-6
In the first step, the condensation reaction was carried out. A methanol solution of 270 g of anhydrous methanol and 500 g of sodium methanolate was added to a 2L four-neck flask. Cooled to 0-5 °C in an ice-water bath, 72.8 g of malononitrile was added and stirred for 30 min, keeping the temperature at 0-5 °C. 146.5 g of N-cyanoimido-S,S-dimethyl dithiocarbonate was added in batches and the reaction was stirred at 0-5 °C for 12 hours. The reaction was completed and filtered and the filter cake was washed with 100 mL of cold anhydrous methanol and drained. The wet product was used directly in the next step of the reaction. In the second step, the cyclization reaction was carried out. The above wet product was dissolved in 860 g of water and added to a four-necked flask. After dissolving with stirring, the ice water bath was cooled to 0-5 °C and 1200 g of industrial hydrochloric acid was added slowly and dropwise for about 4-5 hours. The reaction was carried out overnight at 0-5°C. Upon completion, the reaction was filtered and the filter cake was washed twice with 10% sodium carbonate solution and dried to give 157 g of dried product. In the third step, the cyclopropylation reaction was carried out. 315 g of the product of the second step, 1000 g of ethanol and 66.7 g of cyclopropylamine were added to a 2L four-necked flask, stirred and heated to reflux. The reaction was cooled to 0-5°C overnight, filtered, and the filter cake was washed, drained, and dried to give 147 g of dried product. Step 4, oxidation reaction. To a 5L four-necked flask were added 414 g of the third step product, 810 g of acetic acid and 5.5 g of sodium tungstate and heated to 50-55 °C. 230g of hydrogen peroxide was added slowly under stirring, and the reaction was carried out at 50-55°C for 4 hours after the addition. After the reaction was completed, the acetic acid was recovered under reduced pressure, and the residue was cooled to below 10°C with 800g of water, filtered, and the filter cake was washed to pH 5-6, drained, and then used directly in the next step of the reaction. The fifth step, ammoniation reaction. Add the product of the fourth step, 610g of ethanol and 1450g of ammonia in a 5L four-necked flask and stir at 20-25°C for 5 hours. After the reaction was completed, it was cooled to 5-10°C, filtered, and the filter cake was washed and dried to obtain 88g of dried product. The 88g of dry product was dissolved in 560mL of N,N-dimethylformamide and heated to 90°C to dissolve. 5g of powdered activated carbon was added, stirred for 10 minutes, filtered, cooled and refrigerated overnight. Filtered on the next day, the filter cake was washed with deionized water, drained and dried to obtain 80g of white powder.

10191-60-3
207 suppliers
$6.00/5g

765-30-0
502 suppliers
$10.00/25g

109-77-3
445 suppliers
$10.00/5g

112636-83-6
213 suppliers
$72.00/100 mg
Yield:112636-83-6 80 g
Reaction Conditions:
Stage #1:malononitrile with sodium methylate in methanol at 0 - 5; for 0.5 h;
Stage #2:dimethyl N-cyanodithioiminocarbonate in methanol at 0 - 5; for 12 h;
Stage #3:CyclopropylamineFurther stages;
Steps:
1 Example 1:The invention relates to a preparation method of a dicyclanil, Include the following steps:
In the first step, the condensation reaction was carried out. Into a 2L four-necked flask was added 270 g of 500 g of anhydrous methanol and sodium methoxide in methanol. Cool the ice water bath, cool to 0-5°C, add 72.8g malononitrile, stir for 30 minutes, keep the temperature at 0-5°C,In batchwise addition of 146.5 g of N-cyanoimino-S,S-dithiocarbonate dimethyl carbonate, the reaction was stirred at 0-5° C. for 12 h, and the reaction was completed and filtered. The filter cake was washed with 100 mL of cold anhydrous methanol and pumped dry. After being used directly for the next reaction without drying;In the second step, the cyclization reaction, the above wet product was dissolved in 860 g of water and added to a four-necked bottle.After stirring and dissolving, the ice water bath is cooled to 0-5°C, and 1200g of industrial hydrochloric acid is added dropwise. It takes about 4-5 hours to complete the addition.Stir at 0-5 ° C overnight, after the reaction is complete, filter, filter cake washed, washed twice with 10% sodium carbonate, dried, dried in an oven to obtain dried product 157g; In the third step, the cyclopropylation reaction was carried out. In the 2L four-necked bottle, 3157 g of the product in the second step, 1000 g of ethanol and 66.7 g of cyclopropylamine were added, stirred, and the mixture was heated to reflux. The reaction was completed overnight and the reaction was cooled to 0-5. °C, filtration, filter cake washed, drained, dried in an oven to obtain dry product 147g;The fourth step, the oxidation reaction, in the 5L four-necked flask is added to the product of the fourth step 4147g, acetic acid 810g, sodium tungstate 5.5g, heated to 50 ~ 55 °C, while stirring with hydrogen peroxide 230g, plus complete in 50-55 °C reaction 4h, the reaction is complete, recovery of acetic acid under reduced pressure, the residue was added water 800g, cooled to below 10 °C, filtered, the filter cake was washed with water to pH 5-6, drained and used directly in the next reaction without drying;The fifth step, the ammoniation reaction, in the 5L four-necked flask, the fourth step product, ethanol 610g and ammonia water 1450g, 20-25 ° C under stirring for 5h, the reaction is complete, cooled to 5-10 ° C, filtration, filter cake washed, Dry in an oven to obtain 88g of dry product. Dissolve 88g of dry product in 560mL of NN-dimethylamide.Heated to 90 ° C, dissolved, added powdered activated carbon 5g, stirred for 10 minutes, filtered, cooled and placed in the refrigerator overnight, the next day, filtered, filter cake washed with deionized water, drained, dried in an oven, white Powder is 80g.
References:
Shaanxi Qiyuan Technology Development Co., Ltd.;ZHANG, SHU FEN;Shanxi Qiyuan Science And Technology Development Co., Ltd.;Zhang Shufen CN107698519, 2018, A Location in patent:Paragraph 0008

18340-56-2
5 suppliers
inquiry

765-30-0
502 suppliers
$10.00/25g

112636-83-6
213 suppliers
$72.00/100 mg