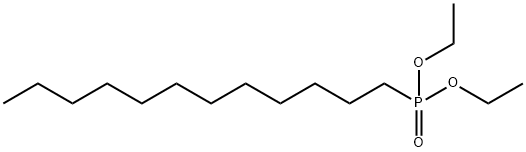
DIETHYL(1-DODECYL)PHOSPHONATE synthesis
- Product Name:DIETHYL(1-DODECYL)PHOSPHONATE
- CAS Number:4844-38-6
- Molecular formula:C16H35O3P
- Molecular Weight:306.42
Yield:4844-38-6 96.3%
Reaction Conditions:
with di-n-pentyl peroxide;silver(I) fluoride at 128; for 5 h;Inert atmosphere;
Steps:
3 Example 3
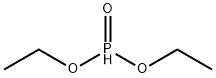
A mixture of 1000 g of diethyl phosphite and 0.61 g of silver fluoride was heated to 128°C with stirring in a 3000 mL reaction flask equipped with a mechanical stirrer, thermometer, peristaltic pump feeder, nitrogen gas duct and condenser. After reaching this temperature, open the peristaltic pump, slowly add 73g initiator dipentyl peroxide and 1010g 1-dodecene to above-mentioned system respectively, and the initiator feeding speed must be controlled to ensure that the reaction system temperature is maintained at 128± 1°C is appropriate. After the feed of the initiator di-p-pentyl peroxide and 1-dodecene was completed, the reaction was continued at 128° C. for 5 hours. The reaction progress was monitored by gas chromatographic analysis, and the end point of the reaction was determined by the disappearance of the chromatographic peak of the raw material 1-dodecene, and the complete conversion to the chromatographic peak of the target product 1-dodecyl phosphonate diethyl ester. The excess diethyl phosphite was removed by distillation under reduced pressure and filtered to obtain the target product, diethyl 1-dodecylphosphonate (purity 99.2% (GC), yield 96.3%).
References:
CN114149463,2022,A Location in patent:Paragraph 0050-0051