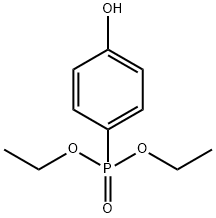
DIETHYL(4-HYDROXYPHENYL)PHOSPHONATE synthesis
- Product Name:DIETHYL(4-HYDROXYPHENYL)PHOSPHONATE
- CAS Number:28255-39-2
- Molecular formula:C10H15O4P
- Molecular Weight:230.2
![Phosphonic acid, P-[4-(acetyloxy)phenyl]-, diethyl ester](/CAS/20210305/GIF/25944-72-3.gif)
25944-72-3
0 suppliers
inquiry

28255-39-2
19 suppliers
inquiry
Yield:28255-39-2 97%
Reaction Conditions:
Stage #1: diethyl p-acetoxyphenylphosphonatewith potassium hydroxide;water in tetrahydrofuran;methanol at 20; for 2 h;
Stage #2: with hydrogenchloride in dichloromethane;water;
Steps:
5
Preparation of p-Diethoxyphosphorylphenol (24). A mixture of 12.02 g (44.17 mmol) of 23 and 4.96 g (88.33 mmol) of KOH in 50 ml of methanol, 50 ml of tetrahydrofuran and 50 ml of water was stirred for 2 hours at RT. The solvents were removed under reduced pressure and the resulting oil treated with 100 ml of DCM and 100 ml of 2N HCl. The organic layer was separated, washed with 2N HCl (1 x 30 ml) , water (2 x 30 ml) , dried overMgSO4 and evaporated under reduced pressure to yield 9.83 g (97%) of 24 as a colourless oil. 1H NMR (CDCl3, 25°C, 600MHz) ?57.66-7.60 (m, 2H, ArH), 7.02-6.97 (m, 2H, ArJi"), 4.14- 4.01 (m, 4H, POCH2), 1.30 (dt, 6H, CH3, J = 6.0/0.6 Hz); 13C NMR (CDCl3, 25°C, 151 MHz) £161.72 (d, 4Jp-C = 3.5 Hz), 133.74 (d, 2Jp-C = 11.6 Hz), 115.97 (d, 3JP-C = 16.4 Hz), 115.65 (d, 1Jp-C = 197.4 Hz), 62.32 (d, 2JP-C - 5.3 Hz), 16.19 (d, 3Jp-C = 6.5 Hz); 31P NMR (CDCl3, 25°C, 243 MHz) ?520.66.
References:
WO2008/148168,2008,A1 Location in patent:Page/Page column 54-55