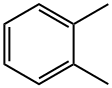
Dinonyl phthalate synthesis
- Product Name:Dinonyl phthalate
- CAS Number:84-76-4
- Molecular formula:C26H42O4
- Molecular Weight:418.61
Yield:-
Reaction Conditions:
Stage #1:o-xylene with oxygen at 200; under 11251.1 Torr; for 0.5 h;
Stage #2:nonyl alcohol at 150; for 2 h;
Steps:
10 Example 10
General procedure: In the continuous reaction apparatus, a mixture of o-xylene, dimethyl phthalate, manganese acetate, cobalt acetate and potassium acetate was poured into an oxidation reaction tube (the reaction mixture had a cobalt, manganese and potassium ion concentration of 300 ppm each, O-xylene concentration of 30%), filled with 3MPa oxygen, 200 ° C under the oxidation, reaction residence time of 2h. The mixture of the oxidized mixture and the methanol was poured into an esterification reaction tube (molecular weight ratio of methanol to starting o-xylene) of 10: 1, The mass ratio of molecular weight of aluminum silicate phosphate to starting o-xylene was 1: 100), and the reaction time was 3h at 150 ° c. After the esterification reaction, the mixture is oxidized to the second methyl group on the benzene ring, and then the esterification reaction is carried out in the esterification reaction tube. After the reaction, the mixture was subjected to gas chromatography and liquid chromatography to calculate the one-way conversion of o-xylene and the selectivity of dimethyl phthalate. The specific practice of Examples 2 to 18 is similar to that of Example 1, and the specific reaction conditions and results are shown in Table 1.
References:
Dalian Institute of Chemical Physics, Chinese Academy of Sciences;XU, JIE;GAO, JIN;HUANG, YIZHENG;MIAO, HONG;MA, HONG CN104418748, 2016, B Location in patent:Paragraph 0020-0023; 0027

111-66-0
146 suppliers
$10.00/10 mL

84-76-4
148 suppliers
$30.00/1g