
Dipyridamole synthesis
- Product Name:Dipyridamole
- CAS Number:58-32-2
- Molecular formula:C24H40N8O4
- Molecular Weight:504.63
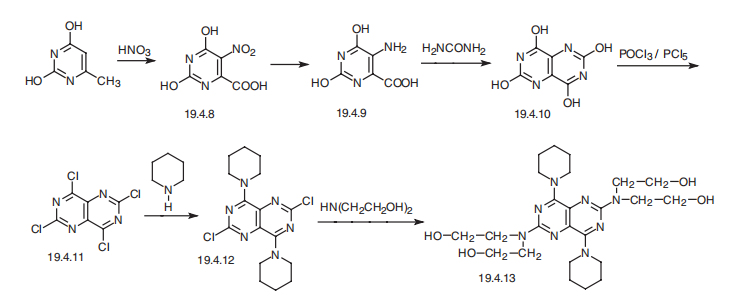
![2,6-Dichloro-4,8-dipiperidinopyrimidino[5,4-d]pyrimidine](/CAS/GIF/7139-02-8.gif)
7139-02-8
114 suppliers
$6.00/250mg
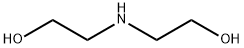
111-42-2
830 suppliers
$10.00/20mg

58-32-2
445 suppliers
$5.00/1g
Yield:58-32-2 95%
Reaction Conditions:
Stage #1:2,6-dichloro-4,8-bis(piperidin-1-yl)pyrimido[5,4-d]pyrimidine;2,2'-iminobis[ethanol] in toluene at 0 - 155; for 9.5 h;
Stage #2: with benzenesulfonic acid in water;toluene at 0 - 95; for 2 h;
Stage #3: with ammonia;pyrographite in ethanol; pH=8 for 0.333333 h;Temperature;
Steps:
1 Example 1, a process for the preparation of dipyridamole
The preparation method described in Example 1 of the present invention was prepared by the following steps:A) The diethanolamine and 2,6-dichloro-4,8-dipiperidinopyrimidino (5,4-D) pyrimidine were charged to the reactor in a weight ratio of 1: (1 to 3) Gradually heated to 155 ° C, the reaction 3h, the mixture;B) to cool to 95 ° C, continue stirring for 1 h; continue to cool to 82 ° C, add the above three times the amount of toluene, stirring 30min; continue to cool to 75 ° C, add the above mixture 12 times the amount of ethanol, stirring 30min; And then cooled to 0 ° C, stirred for 3 h, filtered, the filter cake was washed twice with purified water, and the mixture was cooled to 26 ° C and stirred for 30 hours. Decompression drying to the weight, the crude;C) The crude product was dissolved in 95% acetic acid solution and stirred for 0.5 h at room temperature. The residue was washed twice with purified water, and the mixture was combined with the filtrate to obtain a mixed solution. The mixture was stirred at room temperature with stirring Sodium 6g, stirring 1h after the filter out of the crystal, at 38 to dry, get a salt;D) The above-mentioned primary salt was dissolved in hot water of 95 ° C in a primary salt of primary salt, and benzenesulfonic acid was added in a weight ratio of benzenesulfonic acid to 1: 0.8, and the temperature was gradually lowered to 0 ° C , Stirring 2h, by filtration, drying was secondary salt;E) The secondary salt was dissolved in 65% ethanol solution of the secondary salt 4 times, the ammonia was adjusted to adjust the pH to 8, add a small amount of activated carbon, stir for 20min, filter, filter cake at room temperature with 65% ethanol solution Washing, washing liquid into the crystallization pot, add distilled water to the liquid turbidity, stirring 30min, cooling crystallization, centrifugation, washing, drying, that was dipridamole refined products.Yield (w / w) = 95%; HPLC purity = 99.3%.
References:
Guangzhou Tonghui Pharmaceutical Co., Ltd;Zhang, Tongli;Cheng, Jie;Zhou, Saidong;Chen, Shengpeng;Shi, Wei CN106380471, 2017, A Location in patent:Paragraph 0027; 0028; 0029; 0030; 0031; 0032; 0033-0056
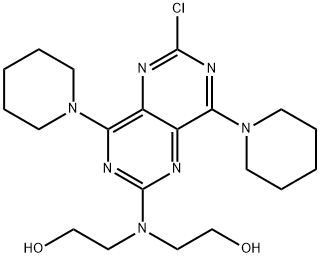
54093-92-4
51 suppliers
$495.42/5MG

111-42-2
830 suppliers
$10.00/20mg

58-32-2
445 suppliers
$5.00/1g
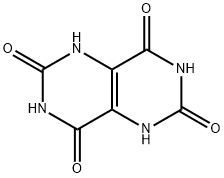
6713-54-8
123 suppliers
$5.00/1g

58-32-2
445 suppliers
$5.00/1g
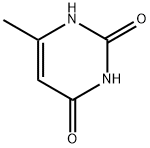
626-48-2
376 suppliers
$10.00/25 g

58-32-2
445 suppliers
$5.00/1g
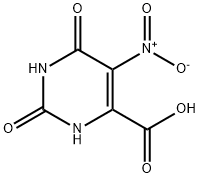
17687-24-0
56 suppliers
inquiry

58-32-2
445 suppliers
$5.00/1g