
Emodin-3-methyl ether synthesis
- Product Name:Emodin-3-methyl ether
- CAS Number:521-61-9
- Molecular formula:C16H12O5
- Molecular Weight:284.26

518-82-1
490 suppliers
$28.00/25mg
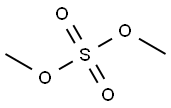
77-78-1
292 suppliers
$22.00/25g

521-61-9
281 suppliers
$28.00/5mg
Yield:521-61-9 81.3%
Reaction Conditions:
with potassium carbonate in acetone at 60; for 12 h;
Steps:
4.2.1. The preparation of physcion 2 (1, 8-dihydroxy-3-methoxy-6-methyl-9,10-anthraquinone)
Emodin 2.499 g (9.25 mmol) and K2CO3 1.341 g (9.70 mmol) were solved in 100 mL acetone. The solution was heated to 60 °C and then a solution of 1 mL dimethyl sulfate in 10 mL acetone was added slowly drop by drop. The mixture was stirred at the reflux temperature for 12 h. Then the reaction was stopped and 50 mL water was added to decompose unreacted dimethyl sulfate. The yellow precipitate was separated from water by filtration and the residue was purified by column chromatography with CH2Cl2/petroleum ether (v/v = 1:1) elution. A yellow solid was obtained.Yield: 81.3%; yellow solid; mp: 208-210 °C (documental value: 205-207 °C [2]); IR (KBr) ν: 3065, 2987, 2840, 1675, 1633, 1567, 1480, 1325, 1166, 980, 875 cm-1; 1H NMR (400 MHz, CDCl3) δ: 12.31 (s, 1.0H, OH), 12.11 (s, 1.0H, OH), 7.63 (d, J = 1.2 Hz, 1.0H, Ar-H), 7.37 (d, J = 2.8 Hz, 1.0H, Ar-H), 7.08 (d,J = 0.8 Hz, 1.0H, Ar-H), 6.69 (d, J = 2.4 Hz, 1.0H, Ar-H), 3.94 (s, 3.0H, OCH3), 2.45 (s, 3.0H, Ar-CH3); ESI-MS m/z 285.1 (M + H)+.
References:
Wang, Wenfeng;Bai, Zedong;Zhang, Fengsen;Wang, Conghui;Yuan, Yaofeng;Shao, Jingwei [European Journal of Medicinal Chemistry,2012,vol. 56,p. 320 - 331]

518-82-1
490 suppliers
$28.00/25mg

74-88-4
343 suppliers
$15.00/10g

521-61-9
281 suppliers
$28.00/5mg
61419-07-6
0 suppliers
inquiry

521-61-9
281 suppliers
$28.00/5mg
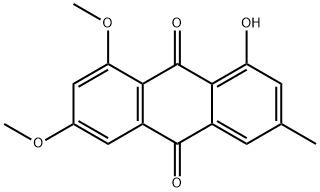
5018-84-8
1 suppliers
inquiry

521-61-9
281 suppliers
$28.00/5mg
![[9,9'-Bianthracene]-10,10'(9H,9'H)-dione, 4,4',5,5'-tetrahydroxy-2,2'-dimethoxy-7,7'-dimethyl-](/CAS/20210305/GIF/21871-90-9.gif)
21871-90-9
3 suppliers
inquiry

521-61-9
281 suppliers
$28.00/5mg