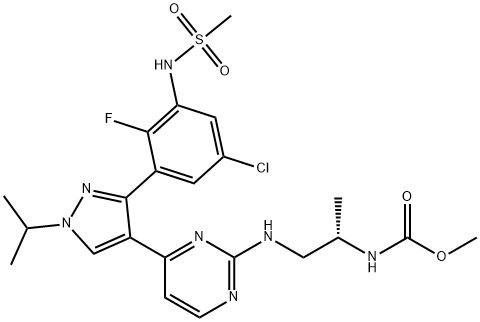
Encorafenib (LGX818) synthesis
- Product Name:Encorafenib (LGX818)
- CAS Number:1269440-17-6
- Molecular formula:C22H27ClFN7O4S
- Molecular Weight:540.01
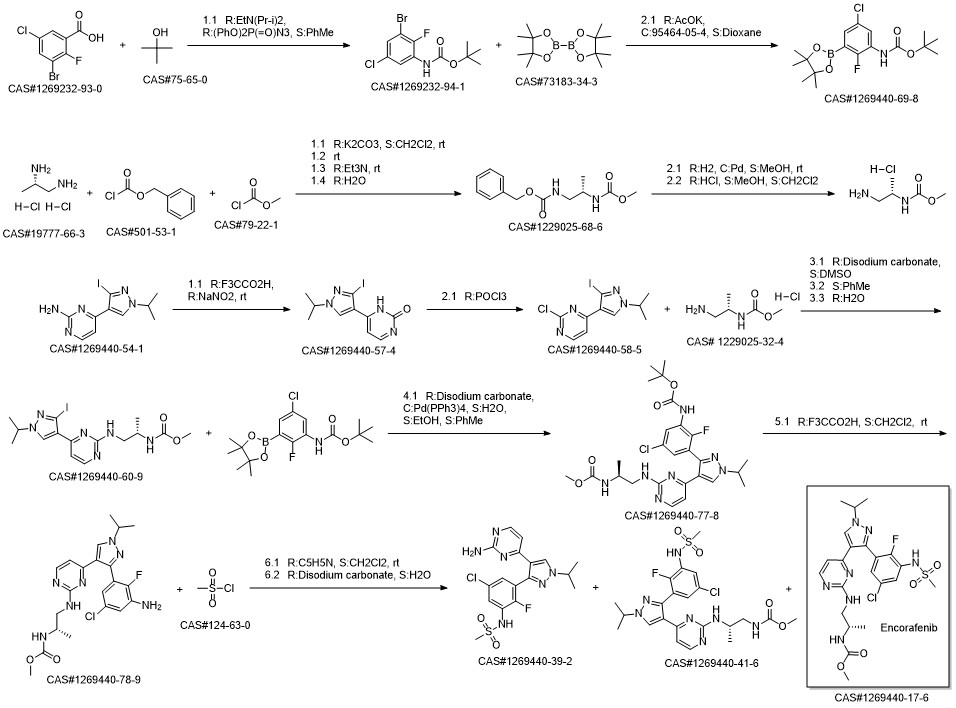
Reference: Huang, Shenlin; Jin, Xianming; Liu, Zuosheng; Poon, Daniel; Tellew, John; Wan, Yongqin; Wang, Xing; Xie, Yongping. Preparation of sulfonamidophenylimidazolylpyrimidine derivatives and analogs for use as protein kinase inhibitors. Assignee IRM LLC, Bermuda; Novartis A.-G. WO 2011025927. (2011).
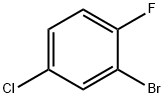
1996-30-1
217 suppliers
$5.00/1g

1269440-17-6
144 suppliers
$50.00/1mg
Yield:-
Steps:
Multi-step reaction with 7 steps
1.1: lithium diisopropyl amide / tetrahydrofuran / 1 h / -78 °C
1.2: -78 - 20 °C
2.1: diphenylphosphoranyl azide; triethylamine / toluene / 2 h / 75 - 84 °C
2.2: 0.25 h / 35 °C
3.1: dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate / 1,4-dioxane / 16 h / 100 °C
4.1: sodium carbonate / tetrakis(triphenylphosphine) palladium(0) / ethanol; water; toluene / 16 h / 80 °C
5.1: trifluoroacetic acid / dichloromethane / 1 h / 20 °C
6.1: triethylamine / 2-methyltetrahydrofuran / -5 - 20 °C
6.2: pH 6 - 6.5
6.3: 0.17 h / 20 °C / pH 7 - 7.5
7.1: sodium hydroxide / 2-methyltetrahydrofuran; water / 0.5 h / 20 - 23 °C
7.2: pH 6 - 6.5
7.3: pH 8.5
References:
IRM LLC;NOVARTIS AG;HUANG, Shenlin;JIN, Xianming;LIU, Zuosheng;POON, Daniel;TELLEW, John;WAN, Yongqin;WANG, Xing;XIE, Yongping WO2011/25927, 2011, A1