
EPROSARTAN synthesis
- Product Name:EPROSARTAN
- CAS Number:133040-01-4
- Molecular formula:C23H24N2O4S
- Molecular Weight:424.51
![METHYL 4-[(2-BUTYL-5-FORMYL-1H-IMIDAZOL-1-YL)METHYL]BENZOATE](/StructureFile/ChemBookStructure22/GIF/CB3507489.gif)
133040-03-6
26 suppliers
inquiry
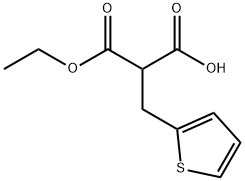
143468-96-6
72 suppliers
$78.00/100mg

133040-01-4
138 suppliers
$49.00/25mg
Yield:133040-01-4 70.92%
Reaction Conditions:
Stage #1:2-n-butyl-1-[(4-carbomethoxyphenyl)methyl]-1H-imidazol-5-carboxaldehyde;3-ethoxy-3-oxo-2-(thien-3-ylmethyl)propanoic acid with piperidine;hydrogenchloride in di-isopropyl ether;water at 40 - 55; under 760.051 Torr;Heating / reflux;
Stage #2: with water;sodium hydroxide in methanol for 3 h;Heating / reflux;
Stage #3: with hydrogenchloride in methanol;water; pH=5.0 - 5.2 at 35 - 40;
Steps:
3
Example 3: Preparation of Eprosartan.11
References:
NEULAND LABORATORIES LTD WO2009/84028, 2009, A2 Location in patent:Page/Page column 7; 11-12
![Methyl (E)-3-[2-Butyl-1-[(4-Carbomethoxyphenyl)methyl]imidazol-5-yl]-2-(2-thienylmethyl)-2-propenoate](/CAS2/GIF/133040-06-9.gif)
133040-06-9
28 suppliers
inquiry

133040-01-4
138 suppliers
$49.00/25mg
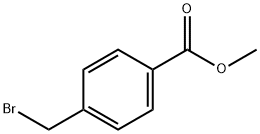
2417-72-3
380 suppliers
$7.00/5g

133040-01-4
138 suppliers
$49.00/25mg
![METHYL 4-[(2-BUTYL-5-FORMYL-1H-IMIDAZOL-1-YL)METHYL]BENZOATE](/StructureFile/ChemBookStructure22/GIF/CB3507489.gif)
133040-03-6
26 suppliers
inquiry

133040-01-4
138 suppliers
$49.00/25mg

83857-96-9
517 suppliers
$8.00/10g

133040-01-4
138 suppliers
$49.00/25mg