
ERYSOLIN synthesis
- Product Name:ERYSOLIN
- CAS Number:504-84-7
- Molecular formula:C6H11NO2S2
- Molecular Weight:193.29
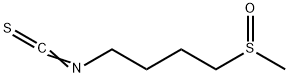
4478-93-7
284 suppliers
$30.00/1mg

504-84-7
24 suppliers
$295.00/50mg
Yield:504-84-7 66%
Reaction Conditions:
with 3-chloro-benzenecarboperoxoic acid in dichloromethane at -20 - 20; for 3 h;Inert atmosphere;
Steps:
15 Oxidation of Sulforaphane
EXAMPLE 15
Oxidation of Sulforaphane
1.77 g of sulforaphane (1 equivalent) obtained in Example 4, as well as 15 mL of dichloromethane are introduced into the reactor, and the mixture is then degassed and set under nitrogen.
1.6 equivalents of meta-chloroperbenzoic acid solubilized in 25 mL of dichloromethane are placed in an isobaric dropping funnel and poured dropwise (within 20 mins) at room temperature, into the reactor.
The addition causes exothermy for producing oxidized sulforaphane i.e. 1-isothiocyanato-4-(methylsulfonyl)butane or 4-methylsulfonylbutyl-isothiocyanate,
Stirring is maintained for 2 hours at room temperature.
A white precipitate forms.
After this period, the reactor is cooled to -20° C. and maintained for 1 hour at this temperature before carrying out filtration.
The filtrate and the solid are analyzed by NMR.
The solid contains derivatives of MCPBA, while the filtrate contains the oxidized product, as well as a few traces of aromatic residues and of residual sulforaphane.
The product is washed with a minimum of a solution saturated with NaHCO3 in order to remove the benzoic acid, and then chromatographed on silica gel, eluent CH2Cl2 100% and then AcOEtT 100%.
After evaporation of the solvents, 1.27 g of a yellowish liquid which solidifies are obtained with a yield of 66%.
TLC: Eluent: AcOEt 100%;
Developer: phosphomolybdic acid;[0261] Rf=0.5. [0262] 1H NMR (300 MHz; CDCl3): [0263] δ (ppm): 3.61 (t, 2H, -CH2-NCS); 3.07 (t, 2H, -S(O)2-CH2); 2.94 (s, 3H, CH3-S(O)2-); 2.07-1.85 (m, 4H, -CH2-CH2-). [0264] 13C NMR (75 MHz; CDCl3): [0265] δ (ppm): 53.63; 44.44; 40.74; 28.55; 19.79. [0266] UV spectrum: [0267] λmax=245 nm [0268] Melting point: 58° C.
References:
US2013/142739,2013,A1 Location in patent:Paragraph 0232-0268

4430-36-8
74 suppliers
$55.00/10mg

504-84-7
24 suppliers
$295.00/50mg

55021-77-7
42 suppliers
$315.00/250mg

504-84-7
24 suppliers
$295.00/50mg
52096-68-1
1 suppliers
inquiry

504-84-7
24 suppliers
$295.00/50mg

27160-24-3
1 suppliers
inquiry

504-84-7
24 suppliers
$295.00/50mg