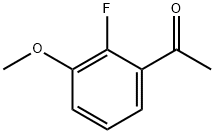
Ethanone, 1-(2-fluoro-3-methoxyphenyl)- (9CI) synthesis
- Product Name:Ethanone, 1-(2-fluoro-3-methoxyphenyl)- (9CI)
- CAS Number:208777-19-9
- Molecular formula:C9H9FO2
- Molecular Weight:168.16
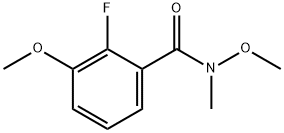
693220-47-2
1 suppliers
inquiry

75-16-1
265 suppliers
$12.00/10ml

208777-19-9
22 suppliers
$45.00/25mg
Yield:-
Reaction Conditions:
Stage #1: 2-fluoro-3,N-dimethoxy-N-methyl-benzamide;methylmagnesium bromide in tetrahydrofuran;diethyl ether at 20; for 2 h;Cooling with ice;
Stage #2: with ammonium chloride in tetrahydrofuran;diethyl ether;water;
Steps:
53.ii
To an ice-cold Compound II (500 mg) in THF (4.7 mL) was added methylmagnesium bromide (1.2 mL, 3.0 M diethyl ether soltuion), and the mixture was stirred for 2 hours while it was allowed to warm up to room temperature. To the reaction mixture was added a saturated aqueous ammonium chloride solution, and the mixture was extracted with ethyl acetate. The organic layer was washed with brine, dried over sodium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluent: hexane/ethyl acetate=4/1) to give Compound III (365 mg).
References:
US2012/225876,2012,A1 Location in patent:Page/Page column 55
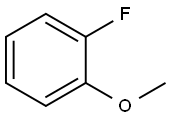
321-28-8
373 suppliers
$6.00/1g
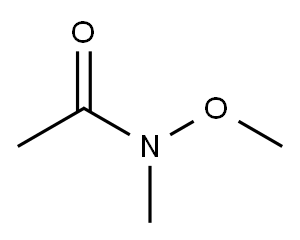
78191-00-1
267 suppliers
$6.00/5g

208777-19-9
22 suppliers
$45.00/25mg
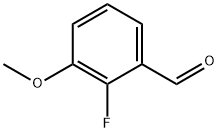
103438-88-6
156 suppliers
$14.14/250mgs:

208777-19-9
22 suppliers
$45.00/25mg
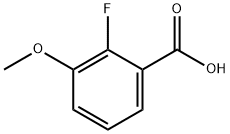
137654-20-7
150 suppliers
$8.00/1g

208777-19-9
22 suppliers
$45.00/25mg