
ETHYL 1-CYCLOPROPYL-1H-IMIDAZOLE-4-CARBOXYLATE synthesis
- Product Name:ETHYL 1-CYCLOPROPYL-1H-IMIDAZOLE-4-CARBOXYLATE
- CAS Number:1001354-64-8
- Molecular formula:C9H12N2O2
- Molecular Weight:180.2
Yield:1001354-64-8 68%
Reaction Conditions:
in butan-1-ol at 100;Microwave irradiation;Sealed tube;
Steps:
43.1 Step 43.1: ethyl 1 -cvclorovl- 1 H-imidazole-4-carboxvlate
A MW vial was charged with cyclopropylamine (3.80 mL, 53.5 mmol) and (Z)-ethyl 3- (dimethylamino)-2-isocyanoacrylate (Step 1.5) (3 g, 17.84 mmol) in n-BuOH (8 mL). The MW vial was sealed and the resulting mixture was heated up and stirred overnight at 100 00 The reaction mixture was diluted with saturated aq. NaHCO3 solution and EtOAc. The aq. layer was separated off and extracted with EtOAc. Combined extracts were dried over Na2504, filtered and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography (heptane/EtOAc 20-100 % EtOAc) to afford the title product (2.24 g, 68 % yield) as orange oil. tR: 0.63 mm (LC-MS 2); ESl-MS: 181 [M+H] (LC-MS 2); TLC (EtOAc) Rf = 0.18.
References:
WO2014/191894,2014,A1 Location in patent:Page/Page column 120; 121

23785-21-9
255 suppliers
$6.00/250mg
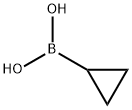
411235-57-9
430 suppliers
$6.00/1g

1001354-64-8
3 suppliers
inquiry
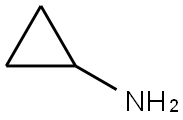
765-30-0
487 suppliers
$10.00/25g

1001354-64-8
3 suppliers
inquiry
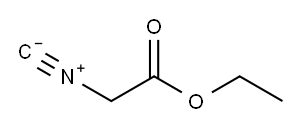
2999-46-4
217 suppliers
$13.43/1gm:

1001354-64-8
3 suppliers
inquiry
