
ethyl 2-(4-hydroxycyclohexyl)acetate synthesis
- Product Name:ethyl 2-(4-hydroxycyclohexyl)acetate
- CAS Number:62141-22-4
- Molecular formula:C10H18O3
- Molecular Weight:186.25
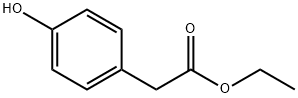
17138-28-2
210 suppliers
$14.00/1g

62141-22-4
26 suppliers
inquiry
Yield:62141-22-4 100%
Reaction Conditions:
with hydrogen;5 weight % Rh on alumina in ethanol; under 3102.97 Torr; for 56 h;
Steps:
1.3.B
Method B; Ethyl (4-hydroxyphenyl)acetate (5Og, 277 mmol) was dissolved in 150 niL of ethanol in a Parr bottle with Rh/Al2O3 (1.00 g, 5 wt%, Aldrich Lot:07727AB). The suspension was hydrogenated on a Parr shaker at 60 psi. After 48 h, starting material was still present, so additional Rh/ Al2O3 (4.00 g) was added. The suspension was hydrogenated for another 8 h, at which time a sample analyzed by 1H NMR indicated the reaction was complete. The reaction mixture was filtered through Celite and rinsed with ethanol (450 mL). The reaction mixture was concentrated to afford a clear colorless oil (55.0 g, quantitative).The resulting ethyl (4-hydroxycyclohexyl)acetate (45.0 g, 240 mmol) was dissolved in AcOH (160 mL) and slowly treated with NaOCl (171 mL, 10-13% available chlorine, -1.7 M, -290 mmol) to maintain the temperature below 30 °C. Brine (500 mL) was added to the solution and the aqueous layer was extracted withEtOAc (3X500 mL). The organic layers were combined, washed with brine (500 mL), concentrated to an oil and chased with heptane. 1H NMR indicated ~1 equivalent of AcOH. The oil was redissolved in EtOAc (500 mL), washed with sat. NaHCO3 (2X250 mL) and brine (200 mL). The organic layer was collected, dried (Na2SO4), filtered, and concentrated to yield 40.7 g (92%) of a clear colorless oil. 1HNMR (DMSO-d6) 64.05 (q, 2H), 2.39 (dt, 2H), 2.30 (d, 2H), 2.19-2.14 (m, 3H), 1.96-1.90 (m, 2H), 1.39 (dq, 2H), 1.18 (t, 3H). GCMS (EI) RT = 9.3 min, [M]+ = 184.
References:
WO2006/44524,2006,A1 Location in patent:Page/Page column 47
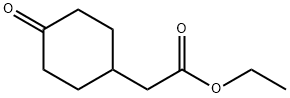
58012-34-3
116 suppliers
$12.00/250mg

62141-22-4
26 suppliers
inquiry

17138-28-2
210 suppliers
$14.00/1g

7440-02-0
417 suppliers
$14.39/1slug

62141-22-4
26 suppliers
inquiry
![1,4-Dioxaspiro[4.5]decan-8-one](/CAS/GIF/4746-97-8.gif)
4746-97-8
489 suppliers
$5.00/1g

62141-22-4
26 suppliers
inquiry
![ethyl 2-(1,4-dioxaspiro[4.5]decan-8-ylidene)acetate](/CAS/GIF/51656-91-8.gif)
51656-91-8
78 suppliers
$37.00/100mg

62141-22-4
26 suppliers
inquiry