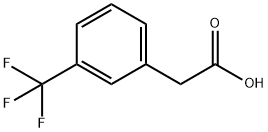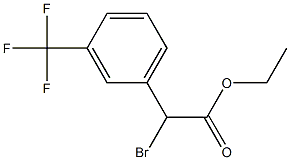
ethyl 2-bromo-2-(3-(trifluoromethyl)phenyl)acetate synthesis
- Product Name:ethyl 2-bromo-2-(3-(trifluoromethyl)phenyl)acetate
- CAS Number:41023-25-0
- Molecular formula:C11H10BrF3O2
- Molecular Weight:311.1
Yield:-
Reaction Conditions:
Stage #1:3-(trifluoromethyl)phenylacetic acid with thionyl chloride;bromineHeating / reflux;
Stage #2:ethanolProduct distribution / selectivity;
Steps:
10
[0192] To a solution of (oc, a, a-trifluoro-m-tolyl) acetic acid 78 (202. 36 g, 0.99 mol) in absolute ethanol (1.0 L) at O °C was added thionyl chloride (79 mL, 1.05 mol), and then the resulting solution was refluxed for 3 h. Concentration in vacuo gave a residue which was partitioned between EtOAc and water. The organic layer was washed with sat. NaHCO3 and brine, dried over Na2S04 and concentrated in vacua to afford 220.1 g of ethyl ester as a pale yellow liquid. [0193] To a mixture of crude ethyl ester (119.15 g, 0.51 mol) and NBS (100.48 g, 0.56 mol) in CC14 (1.0 L) was added benzoyl peroxide (1.0 g). The resulting mixture was heated at 75 °C for 20 min. and then refluxed at 90 °C overnight (14 h) until the brown mixture was turned to a pale-color with-white precipitate. The mixture was cooled to 0 °C, filtered through a pad of celite, concentrated ifa vacuo to afford 151.27 g (95%) of bromide 79 as a pale brown liquid. This product was sufficiently pure to be used directly in subsequent substitute reaction. This product was also prepared by refluxing (a, oc, a-trifluoro-m- tolyl) acetic acid 78 with bromine in the presence of SOC12, and then quenching with EtOH. 1H NMR (400 MHz, CDC13) : 8 7.80 (1H, s), 7.77 (1H, d), 7.61 (1H, d, ), 7.51 (1H, t), 5.35 (1H, s), 4.26 (2H, q), 1.30 (3H, t) ppm.
References:
METABOLEX, INC. WO2005/80340, 2005, A1 Location in patent:Page/Page column 53
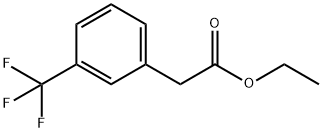
331-33-9
38 suppliers
$45.00/1g

41023-25-0
8 suppliers
inquiry