
Ethyl 2-ethoxynicotinate synthesis
- Product Name:Ethyl 2-ethoxynicotinate
- CAS Number:15441-51-7
- Molecular formula:C10H13NO3
- Molecular Weight:195.22
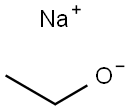
141-52-6
462 suppliers
$10.00/5g

1452-94-4
249 suppliers
$15.00/10g

15441-51-7
14 suppliers
$70.00/500mg
Yield:15441-51-7 65%
Reaction Conditions:
in ethanol at 20; for 12 h;
Steps:
1
EXAMPLE1 cis-4-[(4,5-Bis-(4-chloro-phenyl)-2-(2-ethoxy-pyridin-3-yl)-4,5-dihydro-imidazole-1-carbonyl]-piperazin-2-one; To a solution of 2-chloronicotinic acid (5 g, 31.7 mmol, Aldrich) in dimethylformamide (50 mL) were added potassium carbonate (6.579 g, 47.6 mmol) and ethyl iodide (3.8 mL, 47.7 mmol), respectively. The reaction mixture was stirred at room temperature for 3 d. Water (50 mL) was added and the product was extracted with ethyl ether (2×75 mL). The combined organic phases were washed with brine (1×50 mL), dried over anhydrous magnesium sulfate. The solids were filtered off, and the filtrate was concentrated under reduced pressure to give 2-chloronicotinic acid ethyl ester as yellow oil. It was then dissolved in ethanol (50 mL) and sodium ethoxide (17.8 mL, 47.6 mmol, 21% in ethanol, Aldrich) was added dropwise. Precipitation was seen as sodium ethoxide was added. The reaction mixture was stirred at room temperature for 12 h. The reaction mixture was concentrated and the residue partitioned between methylene chloride (150 mL) and water (50 mL). The aqueous layer was extracted with methylene chloride (1×50 mL). The combined organic layers were washed with brine (1×20 mL) and dried over anhydrous magnesium sulfate. The solids were filtered off, and the filtrate was concentrated in vacuo. The residue was purified by flash chromatography (120 g of silica gel, 10-30% ethyl acetate in hexane) to give 2-ethoxynicotinic acid ethyl ester as an orange oil (4.04 g, 65% yield for 2 steps).
References:
US2007/167437,2007,A1 Location in patent:Page/Page column 6

40134-18-7
227 suppliers
$6.00/5g

64-17-5
715 suppliers
$10.00/50g

141-52-6
462 suppliers
$10.00/5g

15441-51-7
14 suppliers
$70.00/500mg
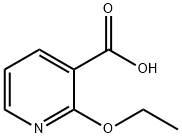
35969-54-1
83 suppliers
$14.14/1gm:

75-03-6
362 suppliers
$11.00/5g

15441-51-7
14 suppliers
$70.00/500mg
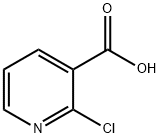
2942-59-8
796 suppliers
$5.00/25g

64-17-5
715 suppliers
$10.00/50g

15441-51-7
14 suppliers
$70.00/500mg

1452-94-4
249 suppliers
$15.00/10g

64-17-5
715 suppliers
$10.00/50g

35969-54-1
83 suppliers
$14.14/1gm:

15441-51-7
14 suppliers
$70.00/500mg