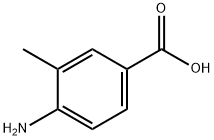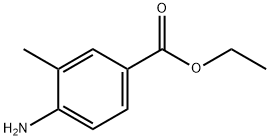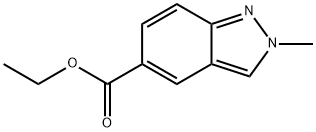
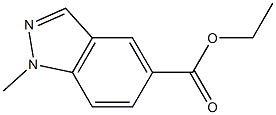
ethyl 2-methyl-2H-indazole-5-carboxylate synthesis
- Product Name:ethyl 2-methyl-2H-indazole-5-carboxylate
- CAS Number:1334405-45-6
- Molecular formula:C11H12N2O2
- Molecular Weight:204.23
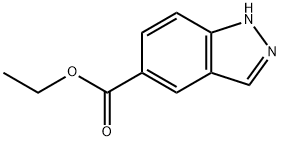
192944-51-7
110 suppliers
$21.00/250mg

74-88-4
342 suppliers
$15.00/10g
1314398-37-2
6 suppliers
inquiry

1334405-45-6
13 suppliers
inquiry
Yield:1314398-37-2 39% ,1334405-45-6 29%
Reaction Conditions:
Stage #1: 1H-indazole-5-carboxylic acid ethyl esterwith sodium hydride in tetrahydrofuran;mineral oil; for 0.166667 h;
Stage #2: methyl iodide in tetrahydrofuran;mineral oil at 20;
Steps:
4.1.3 Ethyl 1-methyl-1H-indazole-5-carboxylate and ethyl 2-methyl-2H-indazole-5-carboxylate
Step 4-1-3
Ethyl 1-methyl-1H-indazole-5-carboxylate and ethyl 2-methyl-2H-indazole-5-carboxylate
Ethyl 1H-indazole-5-carboxylate (1.62 g, 10.0 mmol) obtained in the Step 4-1-2 was dissolved in tetrahydrofuran (20 ml).
To the solution, 60% sodium hydride suspension in oil (420 mg, 10.5 mmol) was added, and the mixture was stirred for 10 minutes.
Methyl iodide (1.49 g, 10.5 mmol) was added dropwise to the mixture.
The resulting mixture was stirred at a room temperature overnight.
Ethyl acetate was added thereto, and the resulting mixture was washed with a saturated sodium bicarbonate solution and then dried over anhydrous magnesium sulfate and concentrated.
The concentrate was purified by silica gel column chromatography (n-hexane:ethyl acetate=3:1) to firstly give ethyl 1-methyl-1H-indazole-5-carboxylate (light-brown solid, 0.80 g, 39%) and to secondly give ethyl 2-methyl-2H-indazole-5-carboxylate (light-brown solid, 0.60 g, 29%).
References:
US2013/79306,2013,A1 Location in patent:Paragraph 0289; 0290; 0291; 0292; 0293; 0294