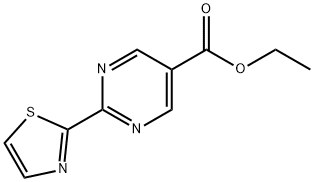
ethyl 2-(thiazol-2-yl)pyrimidine-5-carboxylate synthesis
- Product Name:ethyl 2-(thiazol-2-yl)pyrimidine-5-carboxylate
- CAS Number:1068975-56-3
- Molecular formula:C10H9N3O2S
- Molecular Weight:235.26
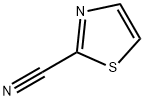
1452-16-0
151 suppliers
$12.00/250mg

76196-81-1
0 suppliers
inquiry

1068975-56-3
6 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: thiazole-2-carbonitrilewith N-Acetylcysteine;ammonium acetate in methanol;Heating / reflux;
Stage #2: C6H7O4(1-)*Na(1+) in N,N-dimethyl-formamide at 100; for 1.5 h;
Steps:
158.1; 158.2
Example 158; 2-Thiazol-2-yl-pyrimidine-5-carboxylic acid (5-fluoro-3-methyl-indol-l-yl)-amide; Step 1 : A flask is charged with 2-cyanothiazole (6.7 g, 60.9 mmol), ammonium acetate (5.63 g, 73 mmol), N-acetylcysteine (993 mg, 6.09 mmol), and methanol (6OmL). The reaction is then heated to reflux overnight. The reaction is reduced in vacuo to yield a residue that is used in the next step without further modifications.
References:
WO2008/121670,2008,A1 Location in patent:Page/Page column 155-156