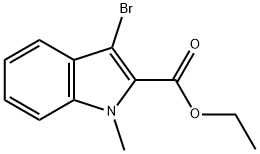
Ethyl 3-bromo-1-methyl-1H-indole-2-carboxylate synthesis
- Product Name:Ethyl 3-bromo-1-methyl-1H-indole-2-carboxylate
- CAS Number:521276-41-5
- Molecular formula:C12H12BrNO2
- Molecular Weight:282.13
91348-45-7
144 suppliers
$12.00/250mg

74-88-4
348 suppliers
$15.00/10g

521276-41-5
19 suppliers
$244.00/500mg
Yield:-
Reaction Conditions:
Stage #1: 3-bromo-1H-indole-2-carboxylic acid ethyl esterwith sodium hydride in N,N-dimethyl-formamide;mineral oil at 20; for 0.333333 h;
Stage #2: methyl iodide in N,N-dimethyl-formamide;mineral oil at 20; for 1 h;
Steps:
Ethyl 3-bromo-l-methyl-lH-indole-2-carboxylate
To a solution of ethyl 3-bromo- lH-indole-2-carboxylate (1.0 g, 3.73 mmol) in dry DMF (20 mL) was added NaH (60%, 1.0 eq, 3.73 mmol) and the mixture stirred for 20 min at rt. Mel (1.0 eq, 3.73 mmol) was then added and the reaction was stirred for 1 h at room temperature. The mixture was added to an aqueous LiCl solution (40 mL) and extracted with EtOAc (20 mL x 2). The combined organic layers were dried over anhydrous Na2SC>4 and concentrated in vacuo to provide ethyl 3- bromo-1 -methyl- lH-indole-2-carboxylate was a light brown oil which was used to the next step without further purification. MS (m/z): Calcd.: 281.0, Found: 282.0 [M + 1]+.
References:
WO2021/216660,2021,A1 Location in patent:Page/Page column 60