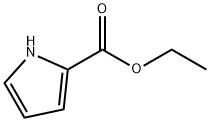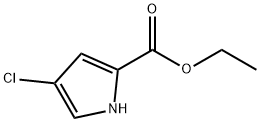
ethyl 4-chloro-1H-pyrrole-2-carboxylate synthesis
- Product Name:ethyl 4-chloro-1H-pyrrole-2-carboxylate
- CAS Number:1254110-74-1
- Molecular formula:C7H8ClNO2
- Molecular Weight:173.6
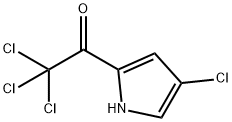
72652-31-4
9 suppliers
$808.88/1G

64-17-5
717 suppliers
$10.00/50g

1254110-74-1
10 suppliers
inquiry
Yield:1254110-74-1 95%
Reaction Conditions:
Stage #1: 2,2,2-trichloro-1-(4-chloro-1H-pyrrol-2-yl)ethan-1-one;ethanolwith sodium at 20; for 0.5 h;
Stage #2: with hydrogenchloride in water;Cooling with ice;
Steps:
7.1
Preparation 7; 4, 6-dichloropyrrolo[l,2-b]pyridazine-3-carboxamideStep 1 : Ethyl 4-[00187] Sodium (0.168 g, 7.29 mmol) was dissolved in anhydrous ethanol (10 mL) then 2,2,2-trichloro-l-(4-chloro-lH-pyrrol-2-yl)ethanone (1.50 g, 6.08 mmol) was added in small portions and the resulting dark brown solution was stirred at room temperature for 30 minutes. The reaction mixture was concentrated under vacuum to dryness and the obtained residue was cooled in an ice bath and 3 N aq. HCl (~ 2 mL) was added slowly and the mixture was extracted with diethyl ether (100 mL x 2). The combined extracts were successively washed with water, sat. aqueous sodium bicarbonate and brine, then dried over magnesium sulfate, filtered and concentrated to afford 1.0 g (95%) of the title compound as a tan oil which solidified upon standing. HPLC (condition B): retention time = 2.71 min. LCMS (condition B): m/z 174 +ve. XH NMR (400 MHz, MeOD) δ ppm 6.97 (1 H, d, J=1.54 Hz), 6.78 (1 H, d, J=1.54 Hz), 4.34 (2 H, q, J=7.19 Hz), 1.39 (3 H, t, J=7.15 Hz).
References:
WO2012/125887,2012,A1 Location in patent:Page/Page column 79-80