
ETHYL (4-CYCLOPROPYLSULFONYLPHENYL)OXOACETATE synthesis
- Product Name:ETHYL (4-CYCLOPROPYLSULFONYLPHENYL)OXOACETATE
- CAS Number:876063-40-0
- Molecular formula:C13H14O5S
- Molecular Weight:282.31

745052-94-2
41 suppliers
$482.00/1g

876063-40-0
23 suppliers
inquiry
Yield:876063-40-0 97.5%
Reaction Conditions:
with 3-chloro-benzenecarboperoxoic acid in dichloromethane at 5 - 20; for 2 h;
Steps:
2
Preparation 2. Ethyl 4-(cyclopropylsulfonyl)phenylglycoxylateCharge a 2-L, three-necked round bottom flask equipped with a mechanical stirrer, addition funnel, and thermometer, with a mixture of ethyl 4-(cyclopropylthio)phenylglyoxilate (80.0 g, 320 mmol) and dichloromethane (640 mL). Cool the solution to +5° C., and add a solution of MCPBA (meta chloro perbenzoic acid) (144.0 g, 640 mmol) in dichloromethane (620 mL) at +5 to +20° C. over 1 hr. Stir the resulting reaction mixture for 1 hr at room temperature. Completion of the reaction can be monitored by GC and TLC (eluent:n-hexane:ethyl acetate=6:4). After the reaction is deemed complete, add 1 L of 2M NaHCO3 over 20 min at 20° C. during which time gas evolution can be observed. Separate the organic and aqueous layers. Extract the aqueous layer with 100 mL of dichloromethane. Combine the organic layers, and sequentially wash the combined organic layers with 300 and 200 mL of water. Evaporate the dichloromethane under reduced pressure to provide 88.1 g (97.5% yield, 95% purity via GC) of the title compound.
References:
US2009/181981,2009,A1 Location in patent:Page/Page column 4; 5
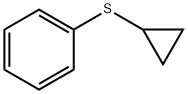
14633-54-6
137 suppliers
$22.00/1g

876063-40-0
23 suppliers
inquiry