
ethyl 4-methyl-2-oxovalerate synthesis
- Product Name:ethyl 4-methyl-2-oxovalerate
- CAS Number:26073-09-6
- Molecular formula:C8H14O3
- Molecular Weight:158.2
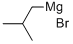
926-62-5
164 suppliers
$154.00/250g
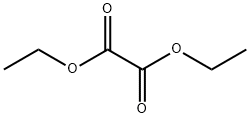
95-92-1
408 suppliers
$5.00/5G

26073-09-6
43 suppliers
$220.00/250mg
Yield:26073-09-6 76%
Reaction Conditions:
in diethyl ether at -78 - 20; for 2 h;Inert atmosphere;
Steps:
Synthesis of ethyl 4-methyl-2-oxopentanoate (4)
730 mg (5 mmol) of diethyl oxalate and 12 mL of anh. diethyl ether were introduced into a 100 mL round-bottom flask under argon atmosphere. The resulting solution was cooled to -78 °C and a solution of the Grignard reagent described above (compound 3, 1.5 eq.) was added. The reaction mixture was stirred at rt for 2 h, after which time the reaction was quenched with NH4Cl 1M and extracted three times with Et2O. The combined organic phases were dried over anh. Na2SO4 and the volatile compounds in the filtrate were removed under reduced pressure. The crude reaction product was purified by column chromatography, yielding 601 mg of compound 4 as a colorless oil in 76% yield. 1H NMR (300 MHz, CDCl3) δ (ppm): 4.28 (q, J=7.2 Hz, 2H, CH2, Et), 2.68 (d, J=6.6 Hz, 2H,CH2iBu), 2.16 (sept, J=6.9 Hz, 1H, CHiBu), 1.33 (t, J=7.2 Hz, 3H, CH3, Et), 0.93 (d, J=6.9 Hz, 6H,2 x CH3iBu); 13C NMR (75 MHz, CDCl3) δ (ppm): 194.5 (C2), 161.5 (C1), 62.4 (CH2, Et), 48.0 (C3, CH2iBu), 24.3 (C4, CHiBu), 22.5 (2 x CH3iBu), 14.1 (CH3, Et); HRMS (ESI): Calcd. for C8H14O3: 158.0943; found: 158.0951.
References:
Bueno, José María;Carda, Miguel;Crespo, Benigno;Cu?at, Ana Carmen;de Cozar, Cristina;León, María Luisa;Marco, J. Alberto;Roda, Nuria;Sanz-Cervera, Juan F. [Bioorganic and Medicinal Chemistry Letters,2016,vol. 26,# 16,p. 3938 - 3944] Location in patent:supporting information

10348-47-7
64 suppliers
$56.00/5G

26073-09-6
43 suppliers
$220.00/250mg
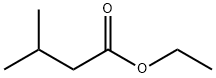
108-64-5
278 suppliers
$10.00/5mL

95-92-1
408 suppliers
$5.00/5G

26073-09-6
43 suppliers
$220.00/250mg
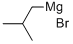
926-62-5
164 suppliers
$154.00/250g

26073-09-6
43 suppliers
$220.00/250mg
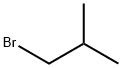
78-77-3
328 suppliers
$14.00/5g

95-92-1
408 suppliers
$5.00/5G

26073-09-6
43 suppliers
$220.00/250mg