
ethyl 6-Methoxy-2-Methylnicotinate synthesis
- Product Name:ethyl 6-Methoxy-2-Methylnicotinate
- CAS Number:173261-76-2
- Molecular formula:C10H13NO3
- Molecular Weight:195.22
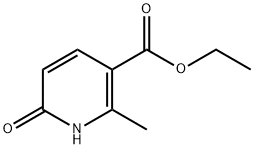
3424-43-9
74 suppliers
$53.00/100mg

74-88-4
340 suppliers
$15.00/10g

173261-76-2
8 suppliers
inquiry
Yield:173261-76-2 75%
Reaction Conditions:
with silver carbonate at 50; for 16 h;
Steps:
Ethyl 6-methoxy-2-methylnicotinate
: To a suspension of ethyl2-methyl-6-oxo-1,6-dihydropyridine-3-carboxylate (0.2 g, 1.06 mmol) and Ag2CO3(0.44 g, 1.59 mmol) was added methyl iodide (0.3 mL, 4.24 mmol)and the reaction mixture was heated at 50 °C for 16 h. The reaction mixture wascooled to room temperature, filtered and concentrated. The crude residue waspurified by Combiflash with 20% EtOAc in Hexane to afford the title compound asoff-white solid (0.25 g, 75%). 1H NMR (DMSO-d6, 400 MHz) δ1.37 (t, J = 7.2 Hz, 3H), 2.75 (s,3H), 3.96 (s, 3H), 4.33 (q, J = 7.2Hz, 2H), 6.57 (d, J = 8.4 Hz, 1H),8.11 (d, J = 8.8 Hz, 1H); MS (ESI) m/z196.1 (M+H)+.
References:
Ruf, Sven;Hallur, Mahanandeesha Siddappa;Anchan, Nisha K.;Swamy, Indu N.;Murugesan, Karthikai Raj;Sarkar, Sayantani;Narasimhulu, Lokesh Kananti;Putta, V.P. Rama Kishore;Shaik, Shama;Chandrasekar, Devaraj Venkatapura;Mane, Vishal Subhash;Kadnur, Sanjay Venkatachalapathi;Suresh, Juluri;Bhamidipati, Ravi Kanth;Singh, Manvi;Burri, Raghunadha Reddy;Kristam, Rajendra;Schreuder, Herman;Czech, Joerg;Rudolph, Christine;Marker, Alexander;Langer, Thomas;Mullangi, Ramesh;Yura, Takeshi;Gosu, Ramachandraiah;Kannt, Aimo;Dhakshinamoorthy, Saravanakumar;Rajagopal, Sridharan [Bioorganic and Medicinal Chemistry Letters,2018,vol. 28,# 5,p. 922 - 925] Location in patent:supporting information
4027-39-8
2 suppliers
inquiry

173261-76-2
8 suppliers
inquiry

10444-33-4
11 suppliers
inquiry

173261-76-2
8 suppliers
inquiry

3424-43-9
74 suppliers
$53.00/100mg

74-88-4
340 suppliers
$15.00/10g

173261-76-2
8 suppliers
inquiry

3424-43-9
74 suppliers
$53.00/100mg

74-88-4
340 suppliers
$15.00/10g

173261-76-2
8 suppliers
inquiry
53256-41-0
1 suppliers
inquiry