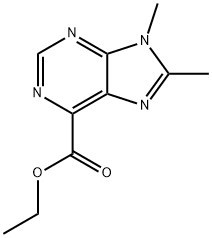
ETHYL 8,9-DIMETHYL-9H-PURINE-6-CARBOXYLATE synthesis
- Product Name:ETHYL 8,9-DIMETHYL-9H-PURINE-6-CARBOXYLATE
- CAS Number:1095823-05-4
- Molecular formula:C10H12N4O2
- Molecular Weight:220.23
Yield:1095823-05-4 9%
Reaction Conditions:
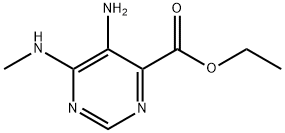
Stage #1: C8H12N4O2;acetyl chloridewith pyridine;triethylamine in dichloromethane at 20;
Stage #2: with acetic acid at 130; for 0.5 h;Microwave irradiation;
Steps:
21
Synthesis of Compound 21.2. A mixture of compound 21.1 (60 mg, 0.30 mmol), acetyl chloride (54 uL, 0.74 mmol), triethylamine (64 uL, 0.46 mmol), pyridine (100 uL 1 mmol), and methylene chloride (1 mL, 0.02 mol) was stirred at RT. MeOH was added to the reaction and the solvent was removed in vacuo. MS m/z 239 [M+1]+. A solution of the residue and acetic acid (2 mL, 0.04 mol) was heated in the microwave at 130° C. for 30 min. The solvent was removed, and the residue was purified by HPLC using water/MeCN with 10 mM NH4HCO3 (C18, 90/10 to 10/90) as eluant to afford compound 21.2 (6 mg, 9%). 1H NMR (400 MHz, DMSO-d6) δ=8.93 (s, 1H), 4.43 (q, J=7.1 Hz, 2H), 3.78 (s, 3H), 2.66 (s, 3H), 1.36 (t, J=7.1 Hz, 3H) MS m/z 221 [M+1]+.
References:
US2009/5359,2009,A1 Location in patent:Page/Page column 28