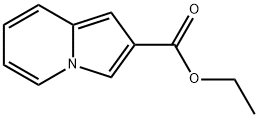
ethyl indolizine-2-carboxylate synthesis
- Product Name:ethyl indolizine-2-carboxylate
- CAS Number:153274-63-6
- Molecular formula:C11H11NO2
- Molecular Weight:189.21
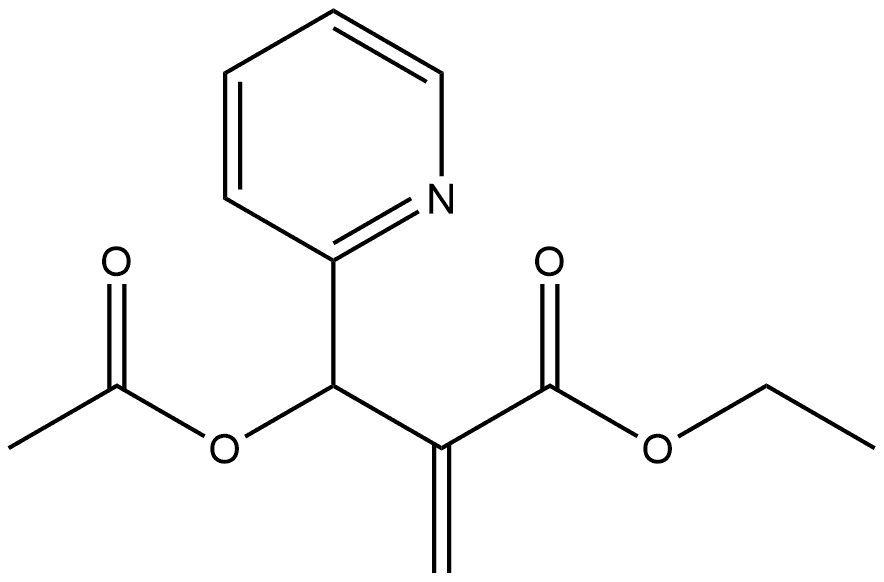
153274-58-9
0 suppliers
inquiry

153274-63-6
6 suppliers
inquiry
Yield:153274-63-6 75%
Reaction Conditions:
at 120; for 0.916667 h;Inert atmosphere;
Steps:
7 Ethyl(indolizine-2-carboxylate) (3)
To a 25 mL flask was added ethyl 2-(acetoxy(pyridin-2- yl)methyl)acrylate (7.0 g, 28 mmol). The acrylate was set to stir at 120 °C under a nitrogen atmosphere. After 55 min, the reaction was judged complete by TLC. The reaction mixture was diluted in 50 mL 5% MeOH: EtOAc and passed through a thick pad of SiO2 using 5% MeOH: EtOAc as eluent. Concentrated to give a dark solid (4.02 g, 21.2 mmol, 75%). 1H NMR (300 MHz, CDCl3) δ 7.85 (d, J= 6.3 Hz, 1.0 Hz, lH), 7.80 (s, 1H), 7.36 (d, J = 9.2 Hz, 1H), 6.83 (s, 1H), 6.68 (dd, J = 9.0 Hz, 6.3 HzlH), 6.53 (td, J= 6.9 Hz, 1,2 Hz, 1H), 4.35 (q, J= 7.1 Hz, 2H), 1.38 (t, J= 7.1 Hz, 3H).
References:
WO2016/19182,2016,A1 Location in patent:Page/Page column 33; 35
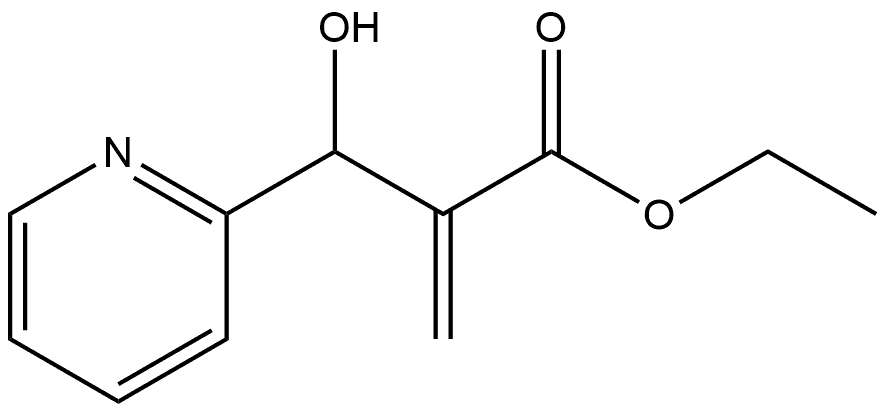
153274-52-3
0 suppliers
inquiry

153274-63-6
6 suppliers
inquiry
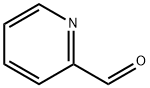
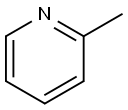
1121-60-4
502 suppliers
$10.60/10gm:

153274-63-6
6 suppliers
inquiry
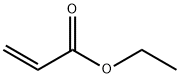
140-88-5
515 suppliers
$12.00/100ml

153274-63-6
6 suppliers
inquiry