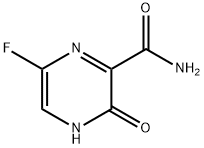
Favipiravir synthesis
- Product Name:Favipiravir
- CAS Number:259793-96-9
- Molecular formula:C5H4FN3O2
- Molecular Weight:157.1

645 grams of sodium hydroxide were dissolved in 9L of water, the temperature was lowered to 5°C, and 1.29 kg of 6-fluoro-3-hydroxy-2-cyanopyrazine was added in batches, stirred, and heated slightly, and the temperature of the reaction system was controlled to -10°C, it takes 3.5 hours to complete the addition, after holding for 1 hour, the temperature is raised to 40°C for 1 hour. Add 100g of activated carbon to the reaction solution, hot filter, cool the mother liquor to 5°C, adjust the pH to 3-4 with concentrated hydrochloric acid, precipitate a large amount of solids, filter and dry to obtain a crude off-white powder, beaten with 2.8 liters of 15% methanol aqueous solution and filter After drying, 1.34 kg of white powder favipiravir was obtained. 1H-NMR (DMSO, 600MHz): δ 13.38 (s, 1H), 8.73 (1s, 1H), 8.51-8.49 (d, J=12, 2H) (yield 91%).
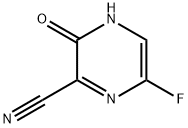
356783-31-8
65 suppliers
inquiry

259793-96-9
504 suppliers
$5.00/50mg
Yield:259793-96-9 92.3%
Reaction Conditions:
Stage #1: 6-fluoro-3-hydroxypyrazine-2-carbonitrilewith sulfuric acid at 50; for 4 h;
Stage #2: with water at 3 - 10; for 0.666667 h;
Stage #3: with sodium hydroxide in water at 10; for 0.75 h;
Steps:
3 Synthesis of 6-fluoro-3-hydroxypyrazine-2-carboxamide
14.39 g of concentrated sulfuric acid was added to 3.45 g of the oily 6-fluoro-3-hydroxypyrazine-2-carbonitrile, and the resulting mixture was stirred at 50° C. for 4 hours. The reaction mixture was then added dropwise over a period of 20 minutes to 44.5 ml of water that had been cooled to 3° C. Following completion of the dropwise addition, the resulting mixture was stirred for 20 minutes at a temperature of not more than 10° C. Subsequently, 10.17 g of a 28% aqueous solution of sodium hydroxide was added dropwise to the reaction mixture over a period of 15 minutes. Following completion of the dropwise addition, the mixture was stirred at the same temperature for 30 minutes. The solid product was then filtered off, and washed with 18 ml of water, yielding 2.22 g (yield: 92.3%) of 6-fluoro-3-hydroxypyrazine-2-carboxamide.
References:
US2011/275817,2011,A1 Location in patent:Page/Page column 5-6
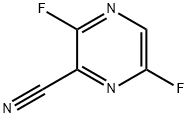
356783-28-3
144 suppliers
inquiry

259793-96-9
504 suppliers
$5.00/50mg
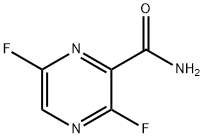
356783-29-4
50 suppliers
inquiry

259793-96-9
504 suppliers
$5.00/50mg
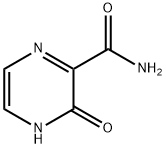
55321-99-8
191 suppliers
$35.39/250mg

259793-96-9
504 suppliers
$5.00/50mg
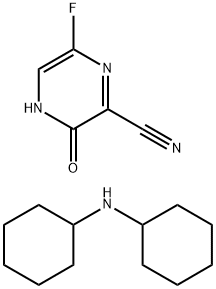
1137606-74-6
50 suppliers
inquiry

259793-96-9
504 suppliers
$5.00/50mg