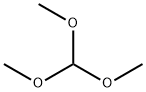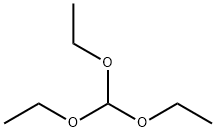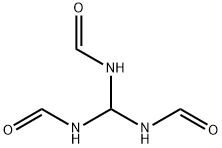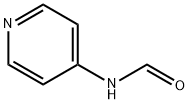
Formamide, N-4-pyridinyl- (9CI) synthesis
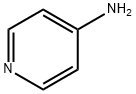
- Product Name:Formamide, N-4-pyridinyl- (9CI)
- CAS Number:22236-91-5
- Molecular formula:C6H6N2O
- Molecular Weight:122.12
Yield:22236-91-5 86%
Reaction Conditions:
with water-soluble Na+ in red mud in methanol at 130; under 15001.5 Torr;Autoclave;Green chemistry;
Steps:
2.3. General procedure for the carbonylation of amines to N-formamides
General procedure: In a typical reaction, the 2 mg of WSS or 50 mg of red mud catalyst isweighed into a 10 mL reactor containing a stir bar. The requiredamounts of morpholine (0.5 mmol), methanol (2 mL) is added. At roomtemperature, the batch reactor is charged with 2.0 MPa of CO. Themixture is stirred at 130 C for 12 h. Afterwards, the autoclave is cooledto room temperature and the pressure is carefully released. The productsof the reaction are analyzed by gas chromatography (GC, Agilent 7890A)and GC-MS spectrometry (MS, Shimadzu). Chromatography purificationsare carried out over diatomite with ethyl acetate as eluent.Analytical data of literature-reported compounds are in accord withreported data. 1H, 13C NMR spectra are recorded on AVANCE NEO400MHZ NMR spectrometer (Bruker). Carbonylation of other amines toN-formamides is carried out with similar procedure.
References:
Chen, Dilong;Ding, Yuxiao;Xia, Chungu;He, Lin;Cao, Yanwei [Molecular catalysis,2022,vol. 533,art. no. 112761]